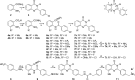Design and Discovery of 2-Arylquinazolin-4-ones as Potent and Selective Inhibitors of Tankyrases - PubMed (original) (raw)
. 2013 Oct 15;4(12):1173-7.
doi: 10.1021/ml400260b. eCollection 2013 Dec 12.
Affiliations
- PMID: 24900625
- PMCID: PMC4027532
- DOI: 10.1021/ml400260b
Design and Discovery of 2-Arylquinazolin-4-ones as Potent and Selective Inhibitors of Tankyrases
Amit Nathubhai et al. ACS Med Chem Lett. 2013.
Abstract
Tankyrases (TNKSs) are poly(ADP-ribose)polymerases (PARPs) that are overexpressed in several clinical cancers. They regulate elongation of telomeres, regulate the Wnt system, and are essential for the function of the mitotic spindle. A set of 2-arylquinazolin-4-ones has been designed and identified as potent and selective TNKS inhibitors, some being more potent and selective than the lead inhibitor XAV939, with IC50 = 3 nM vs. TNKS-2. Methyl was preferred at the 8-position and modest bulk at the 4-position of the 2-phenyl group; electronic effects and H-bonding were irrelevant, but charge in the 4'-substituent must be avoided. Molecular modeling facilitated initial design of the compounds and rationalization of the SAR of binding into the nicotinamide-binding site of the target enzymes. These compounds have potential for further development into anticancer drugs.
Keywords: PARP; Tankyrase; hydrophobic pocket; quinazolin-4-one; selectivity.
Figures
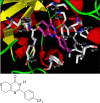
Figure 1
Structure of XAV939 1 and representation of the X-ray crystal structure of 1 bound into the NAD+-binding catalytic domain of human tankyrase-2. Key protein residues are in gray, and key H-bond interactions are shown as green dashed lines.
Scheme 1. Syntheses of Target 2-Arylquinazolin-4-ones 3, 7, and 11 and Locant Numbers
Reagents and conditions: i, PhCHO, NaHSO3, AcNMe2, 150 °C, open flask; ii, CDI; iii, aq. NH3; iv, ArCOCl, pyridine; v, aq. NaOH (0.5 M), 60°C; vi, +NH4HCO2–, Pd/C, MeOH, DMF; vii, BBr3, CH2Cl2 reflux; viii, CbzCl, aq. K2CO3; ix, SOCl2; x, 5a, pyridine; xi, aq. K2CO3 (1.0 M), 100°C; xii, HBr, AcOH.
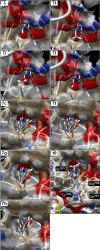
Figure 2
Molecular modeling of 2-arylquinazolin-4-one inhibitors into the structure of the nicotinamide-binding site of tankyrase-2. The upper four panels compare the binding of the 8-substituent while keeping the 2-aryl group constant. The lower five panels show the location of the 2-aryl group in the hydrophobic pocket, placing the 4′-substituent in a narrower tunnel through to solvent water.
Similar articles
- Structure-activity relationships of 2-arylquinazolin-4-ones as highly selective and potent inhibitors of the tankyrases.
Nathubhai A, Haikarainen T, Hayward PC, Muñoz-Descalzo S, Thompson AS, Lloyd MD, Lehtiö L, Threadgill MD. Nathubhai A, et al. Eur J Med Chem. 2016 Aug 8;118:316-27. doi: 10.1016/j.ejmech.2016.04.041. Epub 2016 Apr 20. Eur J Med Chem. 2016. PMID: 27163581 - Exploration of the nicotinamide-binding site of the tankyrases, identifying 3-arylisoquinolin-1-ones as potent and selective inhibitors in vitro.
Paine HA, Nathubhai A, Woon EC, Sunderland PT, Wood PJ, Mahon MF, Lloyd MD, Thompson AS, Haikarainen T, Narwal M, Lehtiö L, Threadgill MD. Paine HA, et al. Bioorg Med Chem. 2015 Sep 1;23(17):5891-908. doi: 10.1016/j.bmc.2015.06.061. Epub 2015 Jul 2. Bioorg Med Chem. 2015. PMID: 26189030 - Structure-based design, synthesis and evaluation in vitro of arylnaphthyridinones, arylpyridopyrimidinones and their tetrahydro derivatives as inhibitors of the tankyrases.
Kumpan K, Nathubhai A, Zhang C, Wood PJ, Lloyd MD, Thompson AS, Haikarainen T, Lehtiö L, Threadgill MD. Kumpan K, et al. Bioorg Med Chem. 2015 Jul 1;23(13):3013-32. doi: 10.1016/j.bmc.2015.05.005. Epub 2015 May 13. Bioorg Med Chem. 2015. PMID: 26026769 - Tankyrases as drug targets.
Lehtiö L, Chi NW, Krauss S. Lehtiö L, et al. FEBS J. 2013 Aug;280(15):3576-93. doi: 10.1111/febs.12320. Epub 2013 Jun 18. FEBS J. 2013. PMID: 23648170 Review. - Tankyrase as a Novel Molecular Target in Cancer and Fibrotic Diseases.
Lakshmi TV, Bale S, Khurana A, Godugu C. Lakshmi TV, et al. Curr Drug Targets. 2017;18(10):1214-1224. doi: 10.2174/1389450117666160715152503. Curr Drug Targets. 2017. PMID: 27425647 Review.
Cited by
- Bismuth-Catalyzed Synthesis of 2-Substituted Quinazolinones.
Vemula SR, Kumar D, Cook GR. Vemula SR, et al. Tetrahedron Lett. 2018 Oct 17;59(42):3801-3805. doi: 10.1016/j.tetlet.2018.09.014. Epub 2018 Sep 7. Tetrahedron Lett. 2018. PMID: 31274936 Free PMC article. - Combining pharmacophore models derived from DNA-encoded chemical libraries with structure-based exploration to predict Tankyrase 1 inhibitors.
Montoya AL, Glavatskikh M, Halverson BJ, Yuen LH, Schüler H, Kireev D, Franzini RM. Montoya AL, et al. Eur J Med Chem. 2023 Jan 15;246:114980. doi: 10.1016/j.ejmech.2022.114980. Epub 2022 Dec 2. Eur J Med Chem. 2023. PMID: 36495630 Free PMC article. - Small-molecule inhibitors of Wnt signaling pathway: towards novel anticancer therapeutics.
Zheng S, Liu J, Wu Y, Huang TL, Wang G. Zheng S, et al. Future Med Chem. 2015;7(18):2485-505. doi: 10.4155/fmc.15.159. Epub 2015 Dec 16. Future Med Chem. 2015. PMID: 26670195 Free PMC article. Review. - Regulation of Wnt/β-catenin signalling by tankyrase-dependent poly(ADP-ribosyl)ation and scaffolding.
Mariotti L, Pollock K, Guettler S. Mariotti L, et al. Br J Pharmacol. 2017 Dec;174(24):4611-4636. doi: 10.1111/bph.14038. Epub 2017 Nov 5. Br J Pharmacol. 2017. PMID: 28910490 Free PMC article. Review. - 2-Phenylquinazolinones as dual-activity tankyrase-kinase inhibitors.
Nkizinkiko Y, Desantis J, Koivunen J, Haikarainen T, Murthy S, Sancineto L, Massari S, Ianni F, Obaji E, Loza MI, Pihlajaniemi T, Brea J, Tabarrini O, Lehtiö L. Nkizinkiko Y, et al. Sci Rep. 2018 Jan 26;8(1):1680. doi: 10.1038/s41598-018-19872-3. Sci Rep. 2018. PMID: 29374194 Free PMC article.
References
- Smith S.; Giriat I.; Schmitt A.; de Lange T. Tankyrase, a poly(ADP-ribose) polymerase at human telomeres. Science 1998, 282, 1484–1487. - PubMed
- Smogorzewska A.; de Lange T. Regulation of telomerase by telomeric proteins. Annu. Rev. Biochem. 2004, 73, 177–208. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Miscellaneous