Discovery of tetrahydropyrazolopyrimidine carboxamide derivatives as potent and orally active antitubercular agents - PubMed (original) (raw)
doi: 10.1021/ml400071a. eCollection 2013 May 9.
Gang Wang 1, Wai Ling Chan 1, Shi Hua Ang 1, Josephine Wong 1, Ida Ma 1, Srinivasa P S Rao 1, Ujjini Manjunatha 1, Suresh B Lakshminarayana 1, Maxime Herve 1, Cyrille Kounde 1, Bee Huat Tan 1, Pamela Thayalan 1, Seow Hwee Ng 1, Mahesh Nanjundappa 1, Sindhu Ravindran 1, Peck Gee 1, Maria Tan 1, Liu Wei 1, Anne Goh 1, Pei-Yu Chen 1, Kok Sin Lee 1, Chen Zhong 2, Trixie Wagner 3, Ina Dix 3, Arnab K Chatterjee 2, Kevin Pethe 2, Kelli Kuhen 2, Richard Glynne 2, Paul Smith 1, Pablo Bifani 1, Jan Jiricek 1
Affiliations
- PMID: 24900693
- PMCID: PMC4027361
- DOI: 10.1021/ml400071a
Discovery of tetrahydropyrazolopyrimidine carboxamide derivatives as potent and orally active antitubercular agents
Fumiaki Yokokawa et al. ACS Med Chem Lett. 2013.
Erratum in
- ACS Med Chem Lett. 2014 Apr 10;5(4):451
Abstract
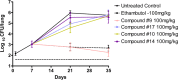
Tetrahydropyrazolo[1,5-a]pyrimidine scaffold was identified as a hit series from a Mycobacterium tuberculosis (Mtb) whole cell high through-put screening (HTS) campaign. A series of derivatives of this class were synthesized to evaluate their structure-activity relationship (SAR) and structure-property relationship (SPR). Compound 9 had a promising in vivo DMPK profile in mouse and exhibited potent in vivo activity in a mouse efficacy model, achieving a reduction of 3.5 log CFU of Mtb after oral administration to infected mice once a day at 100 mg/kg for 28 days. Thus, compound 9 is a potential candidate for inclusion in combination therapies for both drug-sensitive and drug-resistant TB.
Keywords: Antituberculosis; structure−activity relationship; structure−property relationship; tetrahydropyrazolo[1,5-a]pyrimidine.
Figures
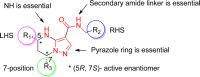
Figure 1
Initial SAR for tetrahydropyrazolopyrimidines.
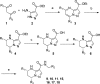
Scheme 1. General Scheme for Synthesis of Tetrahydropyrazolopyrimidines
Reagents and conditions: (a) AcOH at 110 °C; (b) NaBH4, EtOH at room temperature; (c) KOH, aq. EtOH at 60 °C; (d) preparative chiral HPLC; (e) HATU, _i-_PrNEt2, DMF.
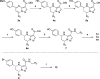
Scheme 2. Synthesis of Tetrahydropyrazolopyrimidines
Reagents and conditions: (a) (i) NaBH4, EtOH at room temperature, (ii) BBr3, CHCl3 at room temperature; (b) KOH, aq. EtOH at 60 °C; (c) preparative chiral HPLC; (d) HATU, _i-_PrNEt2, DMF; (e) 2-methoxy-bromoethane, Cs2CO3, DMF (for 13 and 19), oxetan-3-yl 4-methylbenzenesulfonate, K2CO3, DMF (for 14); (f) morpholine, Pd(OAc)2, Xantphos, Cs2CO3, toluene (for 12).
Figure 2
In vivo efficacy results in TB mouse model.
Similar articles
- Identification of a novel inhibitor of isocitrate lyase as a potent antitubercular agent against both active and non-replicating Mycobacterium tuberculosis.
Liu Y, Zhou S, Deng Q, Li X, Meng J, Guan Y, Li C, Xiao C. Liu Y, et al. Tuberculosis (Edinb). 2016 Mar;97:38-46. doi: 10.1016/j.tube.2015.12.003. Epub 2016 Jan 6. Tuberculosis (Edinb). 2016. PMID: 26980494 - Design, synthesis, and bioevaluation of a novel class of (E)-4-oxo-crotonamide derivatives as potent antituberculosis agents.
Ren J, Xu J, Zhang G, Xu C, Zhao L, You X, Wang Y, Lu Y, Yu L, Wang J. Ren J, et al. Bioorg Med Chem Lett. 2019 Feb 15;29(4):539-543. doi: 10.1016/j.bmcl.2019.01.001. Epub 2019 Jan 3. Bioorg Med Chem Lett. 2019. PMID: 30630715 - Discovery of the disubstituted oxazole analogues as a novel class anti-tuberculotic agents against MDR- and XDR-MTB.
Li D, Gao N, Zhu N, Lin Y, Li Y, Chen M, You X, Lu Y, Wan K, Jiang JD, Jiang W, Si S. Li D, et al. Bioorg Med Chem Lett. 2015 Nov 15;25(22):5178-81. doi: 10.1016/j.bmcl.2015.09.072. Epub 2015 Oct 1. Bioorg Med Chem Lett. 2015. PMID: 26459210 - [Development of antituberculous drugs: current status and future prospects].
Tomioka H, Namba K. Tomioka H, et al. Kekkaku. 2006 Dec;81(12):753-74. Kekkaku. 2006. PMID: 17240921 Review. Japanese. - Derivatives of 3-isoxazolecarboxylic acid esters: a potent and selective compound class against replicating and nonreplicating Mycobacterium tuberculosis.
Lilienkampf A, Pieroni M, Franzblau SG, Bishai WR, Kozikowski AP. Lilienkampf A, et al. Curr Top Med Chem. 2012;12(7):729-34. doi: 10.2174/156802612799984544. Curr Top Med Chem. 2012. PMID: 22283815 Review.
Cited by
- NMR-Verified Dearomatization of 5,7-Substituted Pyrazolo[1,5-a]pyrimidines.
Novikova D, Al Mustafa A, Grigoreva T, Vorona S, Selivanov S, Tribulovich V. Novikova D, et al. Molecules. 2023 Sep 12;28(18):6584. doi: 10.3390/molecules28186584. Molecules. 2023. PMID: 37764360 Free PMC article. - Highly enantioselective Rh-catalyzed asymmetric reductive dearomatization of multi-nitrogen polycyclic pyrazolo[1,5-a]pyrimidines.
Xie C, Xiao G, Guo Q, Wu X, Zi G, Ding W, Hou G. Xie C, et al. Chem Sci. 2023 Jul 28;14(34):9048-9054. doi: 10.1039/d3sc02086j. eCollection 2023 Aug 30. Chem Sci. 2023. PMID: 37655036 Free PMC article. - Comprehensive physicochemical, pharmacokinetic and activity profiling of anti-TB agents.
Lakshminarayana SB, Huat TB, Ho PC, Manjunatha UH, Dartois V, Dick T, Rao SP. Lakshminarayana SB, et al. J Antimicrob Chemother. 2015 Mar;70(3):857-67. doi: 10.1093/jac/dku457. Epub 2014 Nov 11. J Antimicrob Chemother. 2015. PMID: 25587994 Free PMC article. - Combining computational methods for hit to lead optimization in Mycobacterium tuberculosis drug discovery.
Ekins S, Freundlich JS, Hobrath JV, Lucile White E, Reynolds RC. Ekins S, et al. Pharm Res. 2014 Feb;31(2):414-35. doi: 10.1007/s11095-013-1172-7. Epub 2013 Oct 17. Pharm Res. 2014. PMID: 24132686 Free PMC article. - Direct Inhibition of MmpL3 by Novel Antitubercular Compounds.
Li W, Stevens CM, Pandya AN, Darzynkiewicz Z, Bhattarai P, Tong W, Gonzalez-Juarrero M, North EJ, Zgurskaya HI, Jackson M. Li W, et al. ACS Infect Dis. 2019 Jun 14;5(6):1001-1012. doi: 10.1021/acsinfecdis.9b00048. Epub 2019 Mar 28. ACS Infect Dis. 2019. PMID: 30882198 Free PMC article.
References
- World Health Organization. Multidrug and Extensively Drug-Resistant TB: 2010 Global Report on Surveillance and Response.
- Ma Z.; Lienhardt C.; McIlleron H.; Nunn A. J.; Wang X. Global tuberculosis drug development pipeline: the need and the reality. Lancet 2010, 375, 2100–2109. - PubMed
- Koul A.; Arnoult E.; Lounis N.; Guillemont J.; Andries K. The challenge of new drug discovery for tuberculosis. Nature 2011, 469, 483–490. - PubMed
- Pethe K.; Sequeira P. C.; Agarwalla S.; Rhee K.; Kuhen K.; Phong W. Y.; Patel V.; Beer D.; Walker J. R.; Duraiswamy J.; Jiricek J.; Keller T. H.; Chatterjee A.; Tan M. P.; Ujjini M.; Rao S. P.; Camacho L.; Bifani P.; Mak P. A.; Ma I.; Barnes S. W.; Chen Z.; Plouffe D.; Thayalan P.; Ng S. H.; Au M.; Lee B. H.; Tan B. H.; Ravindran S.; Nanjundappa M.; Lin X.; Goh A.; Lakshminarayana S. B.; Shoen C.; Cynamon M.; Kreiswirth B.; Dartois V.; Peters E. C.; Glynne R.; Brenner S.; Dick T. A chemical genetic screen in Mycobacterium tuberculosis identifies carbon-source-dependent growth inhibitors devoid of in vivo efficacy. Nat. Commun. 2010, 1, 57. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous