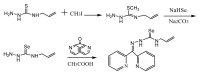Activation of the MAPK11/12/13/14 (p38 MAPK) pathway regulates the transcription of autophagy genes in response to oxidative stress induced by a novel copper complex in HeLa cells - PubMed (original) (raw)
Activation of the MAPK11/12/13/14 (p38 MAPK) pathway regulates the transcription of autophagy genes in response to oxidative stress induced by a novel copper complex in HeLa cells
Wu Zhong et al. Autophagy. 2014 Jul.
Abstract
Transition metal copper (Cu) can exist in oxidized or reduced states in cells, leading to cytotoxicity in cancer cells through oxidative stress. Recently, copper complexes are emerging as a new class of anticancer compounds. Here, we report that a novel anticancer copper complex (HYF127c/Cu) induces oxidative stress-dependent cell death in cancer cells. Further, transcriptional analysis revealed that oxidative stress elicits broad transcriptional changes of genes, in which autophagy-related genes are significantly changed in HYF127c/Cu-treated cells. Consistently, autophagy was induced in HYF127c/Cu-treated cells and inhibitors of autophagy promoted cell death induced by HYF127c/Cu. Further analysis identified that the MAPK11/12/13/14 (formerly known as p38 MAPK) pathway was also activated in HYF127c/Cu-treated cells. Meanwhile, the MAPK11/12/13/14 inhibitor SB203580 downregulated autophagy by inhibiting the transcription of the autophagy genes MAP1LC3B, BAG3, and HSPA1A, and promoted HYF127c/Cu-induced cell death. These data suggest that copper-induced oxidative stress will induce protective autophagy through transcriptional regulation of autophagy genes by activation of the MAPK11/12/13/14 pathway in HeLa cells.
Keywords: HYF127c/Cu; MAPK11; MAPK12; MAPK13; MAPK14; autophagy; copper; oxidative stress; p38 mitogen-activated protein kinase; transcription.
Figures
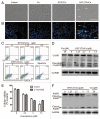
Figure 1. HYF127c/Cu induces cancer cell death and inhibits tumor growth in vivo. (A) The molecular structure of HYF127c (4-allyl-3-selenosemicarbazide). (B) Effect of HYF127c/Cu on the survival of HeLa cells. HeLa cells were exposed for 12 h to different concentrations of HYF127c, CuCl2, or HYF127c/Cu. Cell viability was determined by MTT assay and expressed as the percentage of survival cells. (C) Effect of HYF127c/Cu on the survival of cancer cells and normal cells. (i) Cellular viability of cancer cells and normal cells treated with different concentrations of HYF127c/Cu for 12 h. Each sample was measured in triplicate. (ii) IC50 of HYF127c/Cu in cancer cells. (D) Effect of HYF127c/Cu on the formation of colonies in HeLa cells. (i) Representative images of colonies treated with different concentrations of HYF127c/Cu. (ii) Statistics of the formation of colonies in HeLa cells treated with different concentrations of HYF127c/Cu (n = 3, *P < 0.05). (E) (i) Effect of HYF127c/Cu on tumor volume in human tumor xenografts. (ii) Effect of HYF127c/Cu on the weights of human tumor xenografts. (iii) Effect of HYF127c/Cu on tumor weight in human tumor xenografts (n = 8, *P < 0.05).
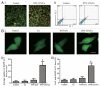
Figure 2. HYF127c/Cu induces apoptosis in HeLa cells. (A) Morphology changes in HeLa cells treated with HYF127c/Cu. Scale bar: 50 μm. (B) Nuclear changes in HeLa cells treated with HYF127c/Cu (arrows indicate the condensation of chromatins). Scale bar: 50 μm. (C) ANXA5-propidium iodide (PI) staining of HeLa cells treated with different concentrations of HYF127c/Cu. (D) Western blot results of CASP3 in HeLa cells treated with different concentrations of HYF127c/Cu. (E) Effect of z-VAD-fmk on cellular viability of HeLa cells treated with of HYF127c/Cu (n = 3, *P < 0.05). (F) Western blot results of PARP1 in HeLa cells treated with different concentrations of HYF127c/Cu.
Figure 3. HYF127c/Cu induces cell death through oxidative stress. (A) Cells were treated with DMSO, 5 μM HYF127c/Cu or an additional 5 mM NAC treatment for 12 h. After incubation with 10 μM H2DCFDA, cells were washed and examined by fluorescence microscopy. Scale bar: 20 μm. (B) Cells were treated with the indicated compounds for 12 h. After incubation with 10 μM H2DCFDA, cells were washed and examined by flow cytometry. (C) Average fluorescence intensity from DCF. (D) Cells were treated with DMSO, 5 μM Cu, 5 μM HYF127c, 5 μM HYF127c/Cu, or 5 μM H2O2 for 12 h. GSH and GSSG were measured with a microplate reader. The relative ratio is shown as indicated (n = 3, *P < 0.05). (E) The relative ratio of cellular viability in cells treated with combinational compounds as indicated (n = 3, *P < 0.05).
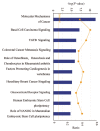
Figure 4. HYF127c/Cu induces broad transcriptional changes of genes in cancer-related pathways. Ingenuity software analysis (IPA) report of RNA-seq data arranged by signaling pathways in order of statistical significance. The top 10 ranked pathways are shown.
Figure 5. HYF127c/Cu induces significant changes of genes involved in hallmarks of cancers or enabling characteristics. Some of the genes significantly regulated by HYF127c/Cu are listed here.
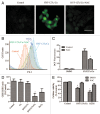
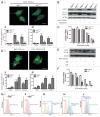
Figure 6. Fluorescent imaging results indicate that HYF127c/Cu induces autophagy in HeLa cells. (A) Accumulation of acidic vesicular organelles in HYF127c/Cu-treated HeLa cells. (i) Representative fluorescence images of cells treated with HYF127c/Cu or the control. In control cells, there was no red fluorescence from AO in the cytoplasm. However, there were many punctate red fluorescing compartments after treatment with 5 μM HYF127c/Cu for 12 h. Scale bar: 50 μm. (ii) Representative FACS results of cells treated with HYF127c/Cu or the control after AO staining. (B) Punctate distribution of EGFP-LC3 in HYF127c/Cu-treated cells. HeLa cells were transfected with EGFP-LC3 24 h before treatment with 5 μM HYF127c/Cu. Twelve h later, the punctate distribution of EGFP-LC3 was visualized and compared with the diffused distribution in control cells (i). Scale bar: 20 µm. The percentage of cells with evident accumulation of EGFP-LC3 dots (ii) and the average number of EGFP-LC3 dots in cells (iii) (n = 3, *P < 0.05).
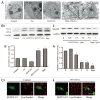
Figure 7. HYF127c/Cu induces autophagy in HeLa cells. (A) Electron microscopy images showing extensive cytoplasm vacuolization enclosed in a double membrane in HYF127c/Cu-treated HeLa cells. Electron microscopy image of an untreated cell is also shown for comparison. The double membrane of the autophagic vacuoles is indicated by a black arrow. N, nucleus; M, mitochondrion. Scale bar: 0.5 μm. (B) Conversion of LC3-I to LC3-II (i and iii) or degradation of SQSTM1 (ii and iv) in HYF127c/Cu-treated cells. HeLa cells were incubated with DMSO, 5 μM Cu, 5 μM HYF127c, 5 μM HYF127c/Cu or 1 μM rapamycin (control) and the amount of endogenous LC3-II proteins or SQSTM1 was analyzed by immunoblot. (C) The fluorescence images showing colocalization of lysosomes and autophagosomes in HYF127c/Cu-treated cells (ii) compared with the control (i). EGFP-LC3-transfected HeLa cells were stained with LysoTracker Red after 20 μM HYF127c/Cu treatment for 12 h. Scale bar: 20 μm.
Figure 8. Inhibition of autophagy promotes HYF127c/Cu-induced cell death. (A) Effect of Atg7 on HYF127c/Cu induced autophagy. Decreased punctate distribution of EGFP-LC3 in _atg7_−/− MEF cells treated with HYF127c/Cu (i). Bar: 20 µm. The average number of EGFP-LC3 dots in cells (ii) and the percentage of cells with evident accumulation of EGFP-LC3 dots (iii). (n = 3, *P < 0.05). (B) Effect of Atg7 on the conversion of LC3-I to LC3-II in HYF127c/Cu-treated cells. Western blots showed that ATG7 deficiency inhibits LC3-I conversion. (C) The effect of Atg7 deficiency on cellular viability, (n = 3, *P < 0.05). (D) CQ inhibits HYF127c/Cu-induced autophagy. (i) Increased punctate distribution of EGFP-LC3 in cells treated with HYF127c/Cu and CQ. Bar: 20 μm. (ii) The percentage of cells with evident accumulation of EGFP-LC3 dots. (iii) The average number of EGFP-LC3 dots in cells. (n = 3, *P < 0.05). (E) Conversion of LC3-I to LC3-II in cells treated with HYF127c/Cu and CQ or HYF127c/Cu alone. Z18, a previously reported autophagy inducer, was used as a control. (F) The effect of the combination of CQ and HYF127c/Cu on cellular viability of HeLa cells. (n = 3, *P < 0.05). (G) Effect of autophagy on HYF127c/Cu-induced oxidative stress indicated by DCF. (i) FACS results of HYF127c/Cu-treated Atg7+/+ MEFs. (ii) FACS results of HYF127c/Cu-treated atg7−/− MEFs. (iii) FACS results of HeLa cells treated with both HYF127c/Cu and CQ. (iv) FACS results of HeLa cells treated with both HYF127c/Cu and 3-MA. (v) FACS results of HeLa cells treated with both HYF127c/Cu and rapamycin.
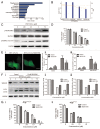
Figure 9. The MAPK11/12/13/14 (p38 MAPK) inhibitor SB203580 decreases HYF127c/Cu-induced autophagy. (A) The function of 652 genes (fold > 1 or fold < −1, P < 0.01) analyzed by DAVID. (B) IPA analysis of phosphorylation pathways possibly involved in HYF127c/Cu-induced autophagy and cell death. (C) Phosphorylation of MAPK11/12/13/14 (p38 MAPK) and its downstream target protein MAPKAPK2 in HYF127c/Cu-treated HeLa cells. (D) Effect of the combination of SB203580 and HYF127c/Cu on cellular viability in HeLa cells, (n = 3, *P < 0.05). (E) Decreased punctate distribution of EGFP-LC3 in cells treated with HYF127c/Cu and SB203580 (i). Bar, 20 μM. The percentage of cells with evident accumulation of EGFP-LC3 dots (ii) and the average number of EGFP-LC3 dots in cells (iii) (n = 3, *P < 0.05). (F) Western blot results of SQSTM1 and LC3 in cells treated with both HYF127c/Cu and SB203580 or treated with HYF127c/Cu alone. (G) The effect of SB203580 on cellular viability of HYF127c/Cu-treated Atg7+/+ and _atg7_−/− cells (n = 3, *P < 0.05).
Figure 10. Analysis of the MAPK11/12/13/14 (p38 MAPK) inhibitor SB203580 on transcription. The effect of SB203580 on the transcription of BAG3, MAP1LC3B, HSPA1A and other genes (SQSTM1, ATG16L1, BECN1, ATG5, MTOR, BCL2L1, MCL1, NEFL2, and MLST8) at different time points (0, 4, 8 and 12 h) (n = 3, *P < 0.05).
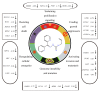
Figure 11. The synthesis of intermediates of 4-allyl-3-selenosemicarbazide.
Similar articles
- The problem of pyridinyl imidazole class inhibitors of MAPK14/p38α and MAPK11/p38β in autophagy research.
Menon MB, Dhamija S, Kotlyarov A, Gaestel M. Menon MB, et al. Autophagy. 2015;11(8):1425-7. doi: 10.1080/15548627.2015.1059562. Autophagy. 2015. PMID: 26061537 Free PMC article. - SB202190-induced cell type-specific vacuole formation and defective autophagy do not depend on p38 MAP kinase inhibition.
Menon MB, Kotlyarov A, Gaestel M. Menon MB, et al. PLoS One. 2011;6(8):e23054. doi: 10.1371/journal.pone.0023054. Epub 2011 Aug 10. PLoS One. 2011. PMID: 21853067 Free PMC article. - p38 MAPK links oxidative stress to autophagy-related gene expression in cachectic muscle wasting.
McClung JM, Judge AR, Powers SK, Yan Z. McClung JM, et al. Am J Physiol Cell Physiol. 2010 Mar;298(3):C542-9. doi: 10.1152/ajpcell.00192.2009. Epub 2009 Dec 2. Am J Physiol Cell Physiol. 2010. PMID: 19955483 Free PMC article. - P38 kinase in gastrointestinal cancers.
Phan T, Zhang XH, Rosen S, Melstrom LG. Phan T, et al. Cancer Gene Ther. 2023 Sep;30(9):1181-1189. doi: 10.1038/s41417-023-00622-1. Epub 2023 May 29. Cancer Gene Ther. 2023. PMID: 37248432 Free PMC article. Review. - Emerging perspectives of copper-mediated transcriptional regulation in mammalian cell development.
Fitisemanu FM, Padilla-Benavides T. Fitisemanu FM, et al. Metallomics. 2024 Oct 4;16(10):mfae046. doi: 10.1093/mtomcs/mfae046. Metallomics. 2024. PMID: 39375833 Free PMC article. Review.
Cited by
- Crosstalk Influence between P38MAPK and Autophagy on Mitochondria-Mediated Apoptosis Induced by Anti-Fas Antibody/Actinomycin D in Human Hepatoma Bel-7402 Cells.
Wang Y, Xia C, Lv Y, Li C, Mei Q, Li H, Wang H, Li S. Wang Y, et al. Molecules. 2017 Oct 17;22(10):1705. doi: 10.3390/molecules22101705. Molecules. 2017. PMID: 29039784 Free PMC article. - Disruption of Copper Redox Balance and Dysfunction under In Vivo and In Vitro Alzheimer's Disease Models.
Xia Y, Tsim KWK, Wang WX. Xia Y, et al. Environ Health (Wash). 2024 Nov 13;3(3):238-249. doi: 10.1021/envhealth.4c00175. eCollection 2025 Mar 21. Environ Health (Wash). 2024. PMID: 40144323 Free PMC article. - Nuclear P38: Roles in Physiological and Pathological Processes and Regulation of Nuclear Translocation.
Maik-Rachline G, Lifshits L, Seger R. Maik-Rachline G, et al. Int J Mol Sci. 2020 Aug 24;21(17):6102. doi: 10.3390/ijms21176102. Int J Mol Sci. 2020. PMID: 32847129 Free PMC article. Review. - Metabolomic Analysis of Mouse Embryonic Fibroblast Cells in Response to Autophagy Induced by Acute Starvation.
Shen S, Weng R, Li L, Xu X, Bai Y, Liu H. Shen S, et al. Sci Rep. 2016 Oct 5;6:34075. doi: 10.1038/srep34075. Sci Rep. 2016. PMID: 27703171 Free PMC article. - Cuproptosis predicts the risk and clinical outcomes of lung adenocarcinoma.
Hu Q, Wang R, Ma H, Zhang Z, Xue Q. Hu Q, et al. Front Oncol. 2022 Aug 8;12:922332. doi: 10.3389/fonc.2022.922332. eCollection 2022. Front Oncol. 2022. PMID: 36003780 Free PMC article.
References
- Loganathan R, Ramakrishnan S, Suresh E, Riyasdeen A, Akbarsha MA, Palaniandavar M. Mixed ligand copper(II) complexes of N,N-bis(benzimidazol-2-ylmethyl)amine (BBA) with diimine co-ligands: efficient chemical nuclease and protease activities and cytotoxicity. Inorg Chem. 2012;51:5512–32. doi: 10.1021/ic2017177. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous