An RNA polymerase II-coupled function for histone H3K36 methylation in checkpoint activation and DSB repair - PubMed (original) (raw)
An RNA polymerase II-coupled function for histone H3K36 methylation in checkpoint activation and DSB repair
Deepak Kumar Jha et al. Nat Commun. 2014.
Erratum in
- Erratum: An RNA polymerase II-coupled function for histone H3K36 methylation in checkpoint activation and DSB repair.
Jha DK, Strahl BD. Jha DK, et al. Nat Commun. 2015 Feb 6;6:6015. doi: 10.1038/ncomms7015. Nat Commun. 2015. PMID: 25655584 No abstract available.
Abstract
Histone modifications are major determinants of DNA double-strand break (DSB) response and repair. Here we elucidate a DSB repair function for transcription-coupled Set2 methylation at H3 lysine 36 (H3K36me). Cells devoid of Set2/H3K36me are hypersensitive to DNA-damaging agents and site-specific DSBs, fail to properly activate the DNA-damage checkpoint, and show genetic interactions with DSB-sensing and repair machinery. Set2/H3K36me3 is enriched at DSBs, and loss of Set2 results in altered chromatin architecture and inappropriate resection during G1 near break sites. Surprisingly, Set2 and RNA polymerase II are programmed for destruction after DSBs in a temporal manner--resulting in H3K36me3 to H3K36me2 transition that may be linked to DSB repair. Finally, we show a requirement of Set2 in DSB repair in transcription units--thus underscoring the importance of transcription-dependent H3K36me in DSB repair.
Figures
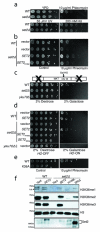
Fig 1. Methylation by Set2 at H3K36 and RNAPII–Set2 interaction is required for cellular resistance against double strand break (DSB)
Five-fold serial dilutions of over-night cultures (grown either in YPD or appropriate selection medium for plasmids) were spotted on indicated plates and pictures were taken after 2-4 days. For immunoblots, log phase cultures were used for preparing whole cell lysates as described in Experimental Procedures. (A) A set2Δ strain is sensitive to phleomycin but not to hydroxyurea and UV. (B) Sensitivity of set2Δ to DSB can be rescued by wild-type SET2 but not by a catalytically dead (SET2H199L) mutant. (C) set2Δ is sensitive to persistent Homothallic (HO) endonuclease-mediated DSB in the “donorless” strain JKM179. Schematic of the “donorless” strain is shown where the Gal-induced DSB is in the MAT locus of chromosome III. This strain has the HML and the HMR deleted and hence the DSB can be repaired only by NHEJ. (D) Rescue of set2Δ sensitivity to persistent DSB by wild-type SET2 but not by a catalytically dead (SET2H199L) or the SRIΔ mutant. (E) H3K36A phenocopies set2Δ on phleomycin. (F) Immunoblots showing the expression from the constructs used in Figs 1B and 1D. All the experiments were replicated at least three times.
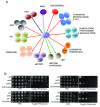
Fig 2. Genetic interaction of SET2 with representative DNA damage response and repair genes
Representative DNA damage response and repair genes were selected from the Open Biosystems gene knockout collection and were verified by either phenotypic analysis or PCRs (using primers specific for the gene deletion) or both. Subsequent double mutants were made by gene replacement. (A) Qualitative representation of genetic interactions (on MMS) of SET2 with DNA damage response and repair genes. Genes have been clustered together, manually, based on their annotated function in the Saccharomyces Genome Database (SGD). Black lines represent no genetic interaction, red lines indicate synthetic sick, and green lines indicate same pathway interaction. The entire screen was repeated twice. (B) Serial dilutions of representative strains show that set2Δ is sick with rad51Δ and asf1Δ while the set2Δ is epistatic to both dot1Δ and yku70Δ on phleomycin. Individual spotting assays were done in triplicate plates (at once) and the set of triplicates were repeated twice.
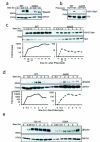
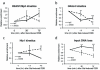
Fig 3. DNA damage signaling is attenuated in set2Δ
Log-phase cells were exposed to phleomycin for indicated times and whole cell extract was prepared (see Methods). (A) Diminution in Rad53 hyper-phosphorylation at indicated time-periods in a set2Δ after phleomycin treatment. The antibody to Rad53 is against the entire protein and G6PDH is used as a loading control. (B) Abrogated γ-H2A.X activation in set2Δ after phleomycin (50 μg/mL) treatment for two hours. (C) Time-course showing the activation of γ-H2A.X after phleomycin treatment. Quantification (H2AS129ph/H2A) of the Western blot is shown below. (D) Activation of Rad53 was monitored after phleomycin treatment in WT and set2Δ. Quantification (Rad53/G6PDH) of the Western blot is shown below, which reveals abrogated DNA damage checkpoint activation in set2Δ (plotted as a semi-log plot). (E) H3K36A mutant cells also show abrogated DNA damage checkpoint activation as monitored by Rad53 activation kinetics. Representative western blots, from at least 3 biological replicates), are presented.
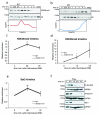
Fig 4. Temporal regulation of H3K36me3 and H3K36me2 correlates with proteasome-mediated degradation of Set2
Immunoblots showing the levels of (A) H3K36me3 and (B) H3K36me2 after DSB induction by phleomycin. Exponentially growing yeast cells were exposed to phleomycin for indicated times and whole cell extract was prepared. (C) H3K36me3 is transiently induced near the site of DSB (assessed by ChIP at 0.2 kb), at early time-points. The error bars represent ± s.e.m from two different biological replicates. n.m. represents ‘not measurable’ (D) H3K36me2 is induced near the site of DSB (assessed by ChIP at 0.2 kb), at later time points. The error bars represent ± s.e.m from two different biological replicates. n.m. represents ‘not measurable’ (* represents p <0.05 for 4C and 4D). (E) Preferential enrichment of Set2 near DSB (assessed by ChIP at 0.2kb from DSB). n.m. represents not measurable (*** represents p<0.01). (F) Time course experiment after phleomycin treatment for Set2 (FLAG-Set2), Ser2pCTD (elongating RNA Polymerase II) and Rpb1 (largest subunit of RNAPII). G6PDH is used as loading control.
Fig 5. Set2 regulates chromatin remodeling after DSB induction
(A) Reduced γ-H2A.X levels in set2Δ by chip. (** represents p<0.03; error bars show ± s.e.m. from two independent biological replicates) (B) Altered kinetics of H4Ac4 at 0.2 kb from the DSB in set2Δ at indicated time points (** represents p <0.03 and *** represents p<0.01; error bars show ± s.e.m. from two independent biological replicates) (C) Increased Htz1 levels at 0.2 kb from the DSB in set2Δ (error bars represent mean ± s.d. from two independent biological replicates). (D) Increased loss of input DNA in set2Δ in G1-arrested cells at 1kb away from the DSB. (** represents p <0.03 and *** represents p<0.01; error bars show ± s.e.m. from two independent biological replicates).
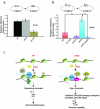
Fig 6. Set2 primarily functions in DSB repair in the context of a transcribing unit
(A) Linearized (SmaI) or circularized pRS316 plasmids were transformed, in parallel, into the indicated strains and colony numbers were counted after 2-3 days on Sc-Ura plates. Religation frequency was calculated as the ratio of the number of colonies obtained from linearized plasmid to the number colonies obtained from circularized plasmid, with the value for WT strain normalized to 1.0. yku70Δ is the positive control for the assay. (B) Linearized (SmaI) or circularized pGV255-live plasmids were transformed, in parallel, into indicated strains and number of colonies were counted after 2-3 days on Sc-Ura plates. Religation frequency was calculated as above. (** represents p< 0.02; error bars represent standard deviation from 3 different independent transformation experiments). (C) A simplified model depicting a function of Set2 and H3K36me in regulating DNA damage response and repair. Immediately after DSB (shown as ‘lightning bolt’), Set2-dependent H3K36me3 becomes enriched around the DSB. Increased H3K36me3 ensures a de-acetylated chromatin structure to prevent inappropriate DNA transactions especially in the context of a transcription unit, which we propose is important for maintaining the genomic integrity of transcribing units. Consistent with this model, loss of Set2/H3K36me leads to abrogated DNA damage signaling activation, altered chromatin structure, and inappropriate repair.
Similar articles
- Shaping the cellular landscape with Set2/SETD2 methylation.
McDaniel SL, Strahl BD. McDaniel SL, et al. Cell Mol Life Sci. 2017 Sep;74(18):3317-3334. doi: 10.1007/s00018-017-2517-x. Epub 2017 Apr 6. Cell Mol Life Sci. 2017. PMID: 28386724 Free PMC article. Review. - Di- and tri-methylation of histone H3K36 play distinct roles in DNA double-strand break repair.
Chen R, Zhao MJ, Li YM, Liu AH, Wang RX, Mei YC, Chen X, Du HN. Chen R, et al. Sci China Life Sci. 2024 Jun;67(6):1089-1105. doi: 10.1007/s11427-024-2543-9. Epub 2024 Feb 29. Sci China Life Sci. 2024. PMID: 38842635 - A histone H3K36 chromatin switch coordinates DNA double-strand break repair pathway choice.
Pai CC, Deegan RS, Subramanian L, Gal C, Sarkar S, Blaikley EJ, Walker C, Hulme L, Bernhard E, Codlin S, Bähler J, Allshire R, Whitehall S, Humphrey TC. Pai CC, et al. Nat Commun. 2014 Jun 9;5:4091. doi: 10.1038/ncomms5091. Nat Commun. 2014. PMID: 24909977 Free PMC article. - Roles for Ctk1 and Spt6 in regulating the different methylation states of histone H3 lysine 36.
Youdell ML, Kizer KO, Kisseleva-Romanova E, Fuchs SM, Duro E, Strahl BD, Mellor J. Youdell ML, et al. Mol Cell Biol. 2008 Aug;28(16):4915-26. doi: 10.1128/MCB.00001-08. Epub 2008 Jun 9. Mol Cell Biol. 2008. PMID: 18541663 Free PMC article. - Structural and functional specificity of H3K36 methylation.
Lam UTF, Tan BKY, Poh JJX, Chen ES. Lam UTF, et al. Epigenetics Chromatin. 2022 May 18;15(1):17. doi: 10.1186/s13072-022-00446-7. Epigenetics Chromatin. 2022. PMID: 35581654 Free PMC article. Review.
Cited by
- H3K36 methyltransferases as cancer drug targets: rationale and perspectives for inhibitor development.
Rogawski DS, Grembecka J, Cierpicki T. Rogawski DS, et al. Future Med Chem. 2016 Sep;8(13):1589-607. doi: 10.4155/fmc-2016-0071. Epub 2016 Aug 22. Future Med Chem. 2016. PMID: 27548565 Free PMC article. Review. - Structural plasticity of methyllysine recognition by the tandem tudor domain of 53BP1.
Tong Q, Cui G, Botuyan MV, Rothbart SB, Hayashi R, Musselman CA, Singh N, Appella E, Strahl BD, Mer G, Kutateladze TG. Tong Q, et al. Structure. 2015 Feb 3;23(2):312-21. doi: 10.1016/j.str.2014.11.013. Epub 2015 Jan 8. Structure. 2015. PMID: 25579814 Free PMC article. - Histone H3 lysine 36 methyltransferase mobilizes NER factors to regulate tolerance against alkylation damage in fission yeast.
Lim KK, Nguyen TTT, Li AY, Yeo YP, Chen ES. Lim KK, et al. Nucleic Acids Res. 2018 Jun 1;46(10):5061-5074. doi: 10.1093/nar/gky245. Nucleic Acids Res. 2018. PMID: 29635344 Free PMC article. - H3K36 dimethylation by MMSET promotes classical non-homologous end-joining at unprotected telomeres.
de Krijger I, van der Torre J, Peuscher MH, Eder M, Jacobs JJL. de Krijger I, et al. Oncogene. 2020 Jun;39(25):4814-4827. doi: 10.1038/s41388-020-1334-0. Epub 2020 May 29. Oncogene. 2020. PMID: 32472076 Free PMC article. - Shaping the cellular landscape with Set2/SETD2 methylation.
McDaniel SL, Strahl BD. McDaniel SL, et al. Cell Mol Life Sci. 2017 Sep;74(18):3317-3334. doi: 10.1007/s00018-017-2517-x. Epub 2017 Apr 6. Cell Mol Life Sci. 2017. PMID: 28386724 Free PMC article. Review.
References
- Hoeijmakers JH. DNA damage, aging, and cancer. N Engl J Med. 2009;361:1475–1485. - PubMed
- Bermejo R, Lai MS, Foiani M. Preventing replication stress to maintain genome stability: resolving conflicts between replication and transcription. Mol Cell. 2012;45:710–718. - PubMed
- Keogh MC, et al. Cotranscriptional set2 methylation of histone H3 lysine 36 recruits a repressive Rpd3 complex. Cell. 2005;123:593–605. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources