Characterization and development of photoactivatable fluorescent proteins for single-molecule-based superresolution imaging - PubMed (original) (raw)
Characterization and development of photoactivatable fluorescent proteins for single-molecule-based superresolution imaging
Siyuan Wang et al. Proc Natl Acad Sci U S A. 2014.
Abstract
Photoactivatable fluorescent proteins (PAFPs) have been widely used for superresolution imaging based on the switching and localization of single molecules. Several properties of PAFPs strongly influence the quality of the superresolution images. These properties include (i) the number of photons emitted per switching cycle, which affects the localization precision of individual molecules; (ii) the ratio of the on- and off-switching rate constants, which limits the achievable localization density; (iii) the dimerization tendency, which could cause undesired aggregation of target proteins; and (iv) the signaling efficiency, which determines the fraction of target-PAFP fusion proteins that is detectable in a cell. Here, we evaluated these properties for 12 commonly used PAFPs fused to both bacterial target proteins, H-NS, HU, and Tar, and mammalian target proteins, Zyxin and Vimentin. Notably, none of the existing PAFPs provided optimal performance in all four criteria, particularly in the signaling efficiency and dimerization tendency. The PAFPs with low dimerization tendencies exhibited low signaling efficiencies, whereas mMaple showed the highest signaling efficiency but also a high dimerization tendency. To address this limitation, we engineered two new PAFPs based on mMaple, which we termed mMaple2 and mMaple3. These proteins exhibited substantially reduced or undetectable dimerization tendencies compared with mMaple but maintained the high signaling efficiency of mMaple. In the meantime, these proteins provided photon numbers and on-off switching rate ratios that are comparable to the best achieved values among PAFPs.
Keywords: PALM; STORM; fPALM; photoconvertible; photoswitchable.
Conflict of interest statement
Conflict of interest statement: A US provisional patent application has been filed for the new fluorescent proteins developed in this work.
Figures
Fig. 1.
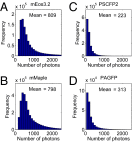
Photon number measurements of PAFPs. The histograms are example photon number distributions of mEos3.2 (A), mMaple (B), PSCFP2 (C), and PAGFP (D) measured by imaging individual Zyxin-PAFP fusion proteins in live BS-C-1 cells. The mean photon numbers are indicated.
Fig. 2.
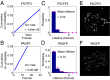
On–off switching rate ratio measurements of PAFPs. Sample data are presented for PSCPF2 (A, C, and E) and PAGFP (B, D, and F). (A and B) Cumulative on-switching probability as a function of time without activation light. The slope of the line gives the on-switching rate. (C and D) Distribution of the on-state lifetime. The blue bars represent measured data, which were fitted with a binned exponential function (magenta dots) Pn=∫n−1n(1/m)e−xmdx, to take into account that lifetimes are rounded to the next larger integer. m is the mean lifetime, the inverse of which gives the off-switching rate. The on–off switching rate ratio is defined as the on-rate divided by the off-rate. Each frame corresponds to 16 ms. (E and F) Representative superresolution images of Zyxin-PSCFP2 and Zyxin-PAGFP in live BS-C-1 cells.
Fig. 3.
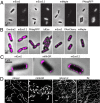
Dimerization tendency of PAFPs and its effect on the distributions of target proteins. (A) Phase contrast (Left) and conventional fluorescent images (Right) of live E. coli cells expressing ClpP-PAFP fusions. PAFPs with substantial dimerization tendencies (mEos2, mMaple) result in the formation of ClpP puncta. PAFPs with little to no dimerization tendencies (mEos3.2, PAtagRFP) produce a diffusive ClpP distribution. (B) Overlaid superresolution (magenta) and phase-contrast (gray) images of live E. coli cells expressing H-NS-PAFP fusions. (C) Overlaid superresolution and phase-contrast images of fixed E. coli cells expressing Tar-PAFP fusions. (D) Superresolution images of fixed Cos-7 cells expressing Vimentin-PAFP fusions or fixed untransfected Cos-7 cells with immunofluorescent labeling of Vimentin (IM). (Scale bars: 1,000 nm.)
Fig. 4.
Signaling efficiency comparison among PAFPs. (A) Superresolution images of live E. coli cells expressing HU-PAFP fusions (magenta) overlaid with phase-contrast images (gray). Superresolution images were acquired until all of the PAFP molecules in the field of view were bleached. (B) Numbers of observed localizations per cell for different HU-PAFP fusions. (Error bars represent SEMs.)
Fig. 5.
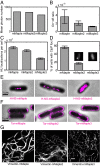
mMaple2 and mMaple3 exhibit both high signaling efficiency and low dimerization tendency. (A–D) Photon budget (A), on–off switching rate ratio (B), signaling efficiency (C), and dimerization tendency (D) of mMaple2 and mMaple3 in comparison with mMaple. (Insets in D) Sample fluorescent images of ClpP-mMaple2– and ClpP-mMaple3–expressing E. coli. Error bars are SEMs and are too small to be visualized in A. (E) H-NS appears more spread out in E. coli cells when fused to mMaple3 in comparison with the mMaple and mMaple2 fusion proteins. (F) Tar-mMaple2 and Tar-mMaple3 appear more evenly distributed along the cell envelope and less concentrated at the polar caps than Tar-mMaple. (G) Vimentin-mMaple2 and Vimentin-mMaple3 are less bundled than Vimentin-mMaple. (Scale bars: 1,000 nm.)
Similar articles
- Higher resolution in localization microscopy by slower switching of a photochromic protein.
Mizuno H, Dedecker P, Ando R, Fukano T, Hofkens J, Miyawaki A. Mizuno H, et al. Photochem Photobiol Sci. 2010 Feb;9(2):239-48. doi: 10.1039/b9pp00124g. Epub 2010 Jan 18. Photochem Photobiol Sci. 2010. PMID: 20126801 - The Role of Probe Photophysics in Localization-Based Superresolution Microscopy.
Pennacchietti F, Gould TJ, Hess ST. Pennacchietti F, et al. Biophys J. 2017 Nov 7;113(9):2037-2054. doi: 10.1016/j.bpj.2017.08.054. Biophys J. 2017. PMID: 29117527 Free PMC article. - Internal rulers to assess fluorescent protein photoactivation efficiency.
Renz M, Wunder C. Renz M, et al. Cytometry A. 2018 Apr;93(4):411-419. doi: 10.1002/cyto.a.23319. Epub 2017 Dec 29. Cytometry A. 2018. PMID: 29286574 - Super-resolution localization microscopy with photoactivatable fluorescent marker proteins.
Hedde PN, Nienhaus GU. Hedde PN, et al. Protoplasma. 2014 Mar;251(2):349-62. doi: 10.1007/s00709-013-0566-z. Epub 2013 Oct 27. Protoplasma. 2014. PMID: 24162869 Review. - Photocontrollable fluorescent proteins for superresolution imaging.
Shcherbakova DM, Sengupta P, Lippincott-Schwartz J, Verkhusha VV. Shcherbakova DM, et al. Annu Rev Biophys. 2014;43:303-29. doi: 10.1146/annurev-biophys-051013-022836. Annu Rev Biophys. 2014. PMID: 24895855 Free PMC article. Review.
Cited by
- STED and STORM Superresolution Imaging of Primary Cilia.
Yang TT, Chong WM, Liao JC. Yang TT, et al. Methods Mol Biol. 2016;1454:169-92. doi: 10.1007/978-1-4939-3789-9_11. Methods Mol Biol. 2016. PMID: 27514922 Free PMC article. - The Growing and Glowing Toolbox of Fluorescent and Photoactive Proteins.
Rodriguez EA, Campbell RE, Lin JY, Lin MZ, Miyawaki A, Palmer AE, Shu X, Zhang J, Tsien RY. Rodriguez EA, et al. Trends Biochem Sci. 2017 Feb;42(2):111-129. doi: 10.1016/j.tibs.2016.09.010. Epub 2016 Nov 1. Trends Biochem Sci. 2017. PMID: 27814948 Free PMC article. Review. - Growth Phase-Dependent Chromosome Condensation and Heat-Stable Nucleoid-Structuring Protein Redistribution in Escherichia coli under Osmotic Stress.
Rafiei N, Cordova M, Navarre WW, Milstein JN. Rafiei N, et al. J Bacteriol. 2019 Nov 5;201(23):e00469-19. doi: 10.1128/JB.00469-19. Print 2019 Dec 1. J Bacteriol. 2019. PMID: 31481544 Free PMC article. - Unraveling the kinetochore nanostructure in Schizosaccharomyces pombe using multi-color SMLM imaging.
Virant D, Vojnovic I, Winkelmeier J, Endesfelder M, Turkowyd B, Lando D, Endesfelder U. Virant D, et al. J Cell Biol. 2023 Apr 3;222(4):e202209096. doi: 10.1083/jcb.202209096. Epub 2023 Jan 27. J Cell Biol. 2023. PMID: 36705602 Free PMC article. - Single-molecule localization to study cytoskeletal structures, membrane complexes, and mechanosensors.
Magrassi R, Scalisi S, Cella Zanacchi F. Magrassi R, et al. Biophys Rev. 2019 Oct;11(5):745-756. doi: 10.1007/s12551-019-00595-2. Epub 2019 Sep 16. Biophys Rev. 2019. PMID: 31529362 Free PMC article. Review.
References
- Betzig E, et al. Imaging intracellular fluorescent proteins at nanometer resolution. Science. 2006;313(5793):1642–1645. - PubMed
- Nienhaus K, Ulrich Nienhaus G. Fluorescent proteins for live-cell imaging with super-resolution. Chem Soc Rev. 2014;43(4):1088–1106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HHMI/Howard Hughes Medical Institute/United States
- GM068518/GM/NIGMS NIH HHS/United States
- GM096450/GM/NIGMS NIH HHS/United States
- R01 GM096450/GM/NIGMS NIH HHS/United States
- R01 GM068518/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous