Constitutive nuclear expression of dentin matrix protein 1 fails to rescue the Dmp1-null phenotype - PubMed (original) (raw)
Constitutive nuclear expression of dentin matrix protein 1 fails to rescue the Dmp1-null phenotype
Shuxian Lin et al. J Biol Chem. 2014.
Abstract
Dentin matrix protein 1 (DMP1) plays multiple roles in bone, tooth, phosphate homeostasis, kidney, salivary gland, reproductive cycles, and the development of cancer. In vitro studies have indicated two different biological mechanisms: 1) as a matrix protein, DMP1 interacts with αvβ3 integrin and activates MAP kinase signaling; and 2) DMP1 serves as a transcription co-factor. In vivo studies have demonstrated its key role in osteocytes. This study attempted to determine whether DMP1 functions as a transcription co-factor and regulates osteoblast functions. For gene expression comparisons using adenovirus constructs, we targeted the expression of DMP1 either to the nucleus only by replacing the endogenous signal peptide with a nuclear localization signal (NLS) sequence (referred to as (NLS)DMP1) or to the extracellular matrix as the WT type (referred to as (SP)DMP1) in MC3T3 osteoblasts. High levels of DMP1 in either form greatly increased osteogenic gene expression in an identical manner. However, the targeted (NLS)DMP1 transgene driven by a 3.6-kb rat Col 1α1 promoter in the nucleus of osteoblasts and osteocytes failed to rescue the phenotyope of Dmp1-null mice, whereas the (SP)DMP1 transgene rescued the rickets defect. These studies support the notion that DMP1 functions as an extracellular matrix protein, rather than as a transcription co-factor in vivo. We also show that DMP1 continues its expression in osteoblasts during postnatal development and that the deletion of Dmp1 leads to an increase in osteoblast proliferation. However, poor mineralization in the metaphysis indicates a critical role for DMP1 in both osteoblasts and osteocytes.
Keywords: Autosomal Recessive Hypophosphatemic Rickets; Biomineralization; Bone; Dentin Matrix Protein 1; Development; Osteoblast; Osteocyte; Osteogenesis; Osteomalacia.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
FIGURE 1.
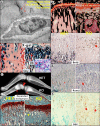
DMP1, highly expressed by osteocytes (Ocy) and also expressed by postnatal osteoblasts (Ob), is likely responsible for regulating osteoblast proliferation and differentiation. a, immuno-gold labeling reveals the presence of DMP1 accumulation in the ECM of the canalicular wall adjacent to the dendrites/cell processes and surrounding the cell body (lacunar wall) of osteocytes. b, X-gal staining of HET long bones displays blue positive cells not only in osteocytes but also in osteoblasts in both embryonic day 16.5 (E16.5; inset) and the postnatal 3 weeks, especially in the metaphysis (right image). c, representative radiographs of 3-week-old long bones reveals the co-existence of an expanded metaphysis (arrows) and a delayed formation of the epiphysis (arrowhead). d and e, histological images showing that in the _Dmp1_-KO tibia metaphysis (right panels), there is a sharp expansion of bone mass with little residual cartilage (d, Safranin O stain, 6 weeks), indicating a lack of endochondral bone formation but the presence of active intramembranous bone formation; von Kossa staining (e, 3 months) shows poor mineralization of the bone (arrow) compared with age-matched control (left). f–h, in situ and immunohistochemical staining showing that in the KO tibia metaphysis (right), there are increased BrdU-positive cells (f), and mRNA levels of Runx2 (g), and OSX (h).
FIGURE 2.
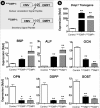
Transfection of Ad-CMV-NLSDMP1 or Ad-CMV-SPDMP1 greatly changes the expression of bone markers in MC3T3 cells. a, schematic constructs of NLSDMP1 and SPDMP1, in which the CMV promoter was used to drive the full-length Dmp1 cDNA. In NLSDMP1, the endogenous DMP1 signal peptide amino acids 1–16 (MKTVILLVFLWGLSCAL) was replaced by the nuclear localization signal peptide (NLS, PPKKKRKV, upper panel) in contrast to the SPDMP1 with its endogenous signal peptide (lower panel). b, >1000-fold increases in NLSDMP1 and SPDMP1 were induced by adenovirus transinduction. There were significant up-regulations of mRNA levels for Bsp, Alp, Ocn, Opn, Dspp, and Sost in both experimental groups with all showing an identical trend. The real-time PCR data, normalized to Gapdh (as an internal control), are presented as mean ± S.E. (n = 4 in each group; *, p < 0.05; **, p < 0.01, compared with the control).
FIGURE 3.
NLSDMP1 localizes to the nucleus and SPDMP1 is secreted into the matrix. a, schematic structure of NLSDMP1 and SPDMP1 transgenes. In the NLSDMP1 construct, the full-length Dmp1 cDNA is driven by the 3.6-kb Col 1_α_1 promoter with its endogenous signal peptide replaced by a NLS peptide, whereas in the SPDMP1 construct, the same 3.6-kb Col 1_α_1 promoter was used to drive the full-length Dmp1 cDNA with its endogenous secretory signal peptide. b, proteins extracted from cell lysate (left) or nuclei (right) of different groups were blotted for the expression level of DMP1. Results show that DMP1 can be found in the cell lysate of all these groups except the _Dmp1_-null group; however, in the nuclear protein extract, it was only detectable in the KO+NLSDMP1 group. c, immunohistochemical staining of DMP1 in 3-week-old mouse long bone. DMP1 is mainly expressed in the HET matrix with little signal in the nucleus, compared with no positive signal in the matrix in KO mice. In the NLSDMP1 transgenic bone, DMP1 is detected in both the matrix and the nucleus of osteocytes (arrows) and osteoblasts (arrowheads), whereas the SPDMP1 is highly expressed in the matrix and the osteoblasts. These data demonstrate that the transgenes are successfully targeted to the nucleus or to the matrix (of bone cells only).
FIGURE 4.
NLSDMP1 does not rescue the rachitic phenotypes in _Dmp1_-null mice; however, SPDMP1 fully rescues the defects. a and b, quantitative data show a greater than 18-fold up-regulation of serum FGF23 levels and a decrease of >20% of the Pi (phosphorus) in _Dmp1_-null mice. The overexpression of NLSDMP1 has no effect on these parameters in either the HET control or KO group; however, the SPDMP1 is fully rescued. d, Safranin O-stained images of 3-week-old tibias, in which there are two expanded regions in the KO growth plate: the proliferation zone (PZ; white lines) and the hypertrophic zone. The NLSDMP1 transgene has no effect in both regions, but SPDMP1 recovers this abnormality. e, BrdU-stained images reveal an increase in the number of BrdU-positive cells in the KO and KO+NLSDMP1 groups. Statistical results demonstrate the above changes in proliferation zone length (c) and cell proliferation numbers (f) in the KO groups, which were significantly different from the control groups., the NLSDMP1 has no apparent effect on the above changes in either the HET or the KO background, but SPDMP1 rescues the KO phenotype. g--j, in situ and immunohistochemical staining shows the molecular changes of Runx2 mRNA (g), sonic hedgehog mRNA (Shh; h), SOX9 (i), and OSX (j). The data indicate an increase in cell proliferation and differentiation in the _Dmp1_-null mice, most likely attributable to hypophosphatemia (data are presented as mean ± S.E.; n = 5; *, p < 0.05; compared with the control). k, representative radiography shows short femurs with poor mineral remodeling in all three _Dmp1_-KO groups (3 weeks, 6 weeks, and 1 year). The targeted expression of NLSDMP1 has no rescue effects on the length, accumulated bone masses, expanded and malformed growth plates (arrows), and distorted tuberosities (arrowheads), all of which are fully rescued by SPDMP1.
FIGURE 5.
The NLSDMP1 transgene has no rescue effects on _Dmp1_-null mice but the SPDMP1 transgene fully rescues the _Dmp1_-null bone phenotype. a–c, compared with SPDMP1, NLSDMP1 has no effect on bone shape and secondary ossification (a, Alizarin red/Alcian blue staining, arrows), bone porosity and mineralization reflected by micro-CT images (b, arrowheads), and Goldner staining (c, red indicating a lack of mineral). d–h, the NLSDMP1 had no apparent effect on the osteocyte (Ocy)-lacunocanalicular system (d, S.E.), and expression of decorin (e, an inhibitory factor in mineralization), OSX (f, a marker for Ob), E11 (g, an early marker for osteocytes), and SOST (h, a marker for mature osteocytes).
FIGURE 6.
The in vivo working model. a, DMP1 secreted from the cell binds to integrin via its RGD domain, followed by activation of the MAP kinase signaling pathway (27, 29). The targeted expression of DMP1 in the nucleus has no direct role in osteogenesis in vivo. b, DMP1 expressed in the osteoblast (Ob) facilitates cell proliferation and transformation (from osteoblasts into osteocytes (Ocy)). Deletion of Dmp1 leads to an increase in cell proliferation and a reduction in osteoblast cell differentiation. Because of the great increase of FGF23 in _Dmp1_-KO osteocytes, the reduced Pi causes an increase in cell proliferation and differentiation in chondrocytes (a new indirect role of hypophosphatemia in the growth plate), in addition to the abnormality in the apoptosis pathway (46).
Similar articles
- Nucleus-targeted Dmp1 transgene fails to rescue dental defects in Dmp1 null mice.
Lin SX, Zhang Q, Zhang H, Yan K, Ward L, Lu YB, Feng JQ. Lin SX, et al. Int J Oral Sci. 2014 Sep;6(3):133-41. doi: 10.1038/ijos.2014.44. Epub 2014 Aug 8. Int J Oral Sci. 2014. PMID: 25105818 Free PMC article. - Nuclear localization of DMP1 proteins suggests a role in intracellular signaling.
Siyam A, Wang S, Qin C, Mues G, Stevens R, D'Souza RN, Lu Y. Siyam A, et al. Biochem Biophys Res Commun. 2012 Aug 3;424(3):641-6. doi: 10.1016/j.bbrc.2012.07.037. Epub 2012 Jul 16. Biochem Biophys Res Commun. 2012. PMID: 22813642 Free PMC article. - Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review. - MMP2-cleavage of DMP1 generates a bioactive peptide promoting differentiation of dental pulp stem/progenitor cell.
Chaussain C, Eapen AS, Huet E, Floris C, Ravindran S, Hao J, Menashi S, George A. Chaussain C, et al. Eur Cell Mater. 2009 Nov 12;18:84-95. doi: 10.22203/ecm.v018a08. Eur Cell Mater. 2009. PMID: 19908197 Free PMC article. - The effectiveness of abstinence-based and harm reduction-based interventions in reducing problematic substance use in adults who are experiencing homelessness in high income countries: A systematic review and meta-analysis: A systematic review.
O'Leary C, Ralphs R, Stevenson J, Smith A, Harrison J, Kiss Z, Armitage H. O'Leary C, et al. Campbell Syst Rev. 2024 Apr 21;20(2):e1396. doi: 10.1002/cl2.1396. eCollection 2024 Jun. Campbell Syst Rev. 2024. PMID: 38645303 Free PMC article. Review.
Cited by
- Skeletal Muscle, but not Cardiovascular Function, Is Altered in a Mouse Model of Autosomal Recessive Hypophosphatemic Rickets.
Wacker MJ, Touchberry CD, Silswal N, Brotto L, Elmore CJ, Bonewald LF, Andresen J, Brotto M. Wacker MJ, et al. Front Physiol. 2016 May 13;7:173. doi: 10.3389/fphys.2016.00173. eCollection 2016. Front Physiol. 2016. PMID: 27242547 Free PMC article. - A novel auditory ossicles membrane and the development of conductive hearing loss in Dmp1-null mice.
Lv K, Huang H, Yi X, Chertoff ME, Li C, Yuan B, Hinton RJ, Feng JQ. Lv K, et al. Bone. 2017 Oct;103:39-46. doi: 10.1016/j.bone.2017.06.007. Epub 2017 Jun 8. Bone. 2017. PMID: 28603080 Free PMC article. - Osteocyte-specific dentin matrix protein 1 : the role of mineralization regulation in low-magnitude high-frequency vibration enhanced osteoporotic fracture healing.
Li MCM, Chow SK, Wong RMY, Chen B, Cheng JCY, Qin L, Cheung WH. Li MCM, et al. Bone Joint Res. 2022 Jul;11(7):465-476. doi: 10.1302/2046-3758.117.BJR-2021-0476.R2. Bone Joint Res. 2022. PMID: 35787000 Free PMC article. - Germline Saturation Mutagenesis Induces Skeletal Phenotypes in Mice.
Rios JJ, Denton K, Russell J, Kozlitina J, Ferreira CR, Lewanda AF, Mayfield JE, Moresco E, Ludwig S, Tang M, Li X, Lyon S, Khanshour A, Paria N, Khalid A, Li Y, Xie X, Feng JQ, Xu Q, Lu Y, Hammer RE, Wise CA, Beutler B. Rios JJ, et al. J Bone Miner Res. 2021 Aug;36(8):1548-1565. doi: 10.1002/jbmr.4323. Epub 2021 May 10. J Bone Miner Res. 2021. PMID: 33905568 Free PMC article. - Inactivation of FAM20B causes cell fate changes in annulus fibrosus of mouse intervertebral disc and disc defects via the alterations of TGF-β and MAPK signaling pathways.
Saiyin W, Li L, Zhang H, Lu Y, Qin C. Saiyin W, et al. Biochim Biophys Acta Mol Basis Dis. 2019 Dec 1;1865(12):165555. doi: 10.1016/j.bbadis.2019.165555. Epub 2019 Sep 9. Biochim Biophys Acta Mol Basis Dis. 2019. PMID: 31513834 Free PMC article.
References
- George A., Sabsay B., Simonian P. A., Veis A. (1993) Characterization of a novel dentin matrix acidic phosphoprotein. Implications for induction of biomineralization. J. Biol. Chem. 268, 12624–12630 - PubMed
- D'Souza R. N., Cavender A., Sunavala G., Alvarez J., Ohshima T., Kulkarni A. B., MacDougall M. (1997) Gene expression patterns of murine dentin matrix protein 1 (Dmp1) and dentin sialophosphoprotein (DSPP) suggest distinct developmental functions in vivo. J. Bone Miner. Res. 12, 2040–2049 - PubMed
- Feng J. Q., Zhang J., Dallas S. L., Lu Y., Chen S., Tan X., Owen M., Harris S. E., MacDougall M. (2002) Dentin matrix protein 1, a target molecule for Cbfa1 in bone, is a unique bone marker gene. J. Bone Miner Res. 17, 1822–1831 - PubMed
- Hirst K. L., Ibaraki-O'Connor K., Young M. F., Dixon M. J. (1997) Cloning and expression analysis of the bovine dentin matrix acidic phosphoprotein gene. J. Dent. Res. 76, 754–760 - PubMed
- MacDougall M., Simmons D., Luan X., Nydegger J., Feng J., Gu T. T. (1997) Dentin phosphoprotein and dentin sialoprotein are cleavage products expressed from a single transcript coded by a gene on human chromosome 4: Dentin phosphoprotein DNA sequence determination. J. Biol. Chem. 272, 835–842 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials