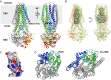Structure of an antibacterial peptide ATP-binding cassette transporter in a novel outward occluded state - PubMed (original) (raw)
Structure of an antibacterial peptide ATP-binding cassette transporter in a novel outward occluded state
Hassanul G Choudhury et al. Proc Natl Acad Sci U S A. 2014.
Abstract
Enterobacteriaceae produce antimicrobial peptides for survival under nutrient starvation. Microcin J25 (MccJ25) is an antimicrobial peptide with a unique lasso topology. It is secreted by the ATP-binding cassette (ABC) exporter McjD, which ensures self-immunity of the producing strain through efficient export of the toxic mature peptide from the cell. Here we have determined the crystal structure of McjD from Escherichia coli at 2.7-Å resolution, which is to the authors' knowledge the first structure of an antibacterial peptide ABC transporter. Our functional and biochemical analyses demonstrate McjD-dependent immunity to MccJ25 through efflux of the peptide. McjD can directly bind MccJ25 and displays a basal ATPase activity that is stimulated by MccJ25 in both detergent solution and proteoliposomes. McjD adopts a new conformation, termed nucleotide-bound outward occluded. The new conformation defines a clear cavity; mutagenesis and ligand binding studies of the cavity have identified Phe86, Asn134, and Asn302 as important for recognition of MccJ25. Comparisons with the inward-open MsbA and outward-open Sav1866 structures show that McjD has structural similarities with both states without the intertwining of transmembrane (TM) helices. The occluded state is formed by rotation of TMs 1 and 2 toward the equivalent TMs of the opposite monomer, unlike Sav1866 where they intertwine with TMs 3-6 of the opposite monomer. Cysteine cross-linking studies on the McjD dimer in inside-out membrane vesicles of E. coli confirmed the presence of the occluded state. We therefore propose that the outward-occluded state represents a transition intermediate between the outward-open and inward-open conformation of ABC exporters.
Keywords: antimicrobial peptide ABC exporter; membrane transporter crystal structure; microcin immunity protein; substrate binding; transport mechanism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
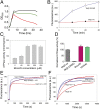
Functional characterization of McjD. (A) Growth of cytotoxic MccJ25-expressing E. coli requires coexpression of ABC exporter McjD (green, production of MccJ25 plus McjD; red, MccJ25 minus McjD; black, without MccJ25 production). (B) Active efflux of fluorescent BODIPY-labeled MccJ25 from cells results in reduced MccJ25 accumulation for McjD compared with the inactive E506Q McjD with equal expression (
Fig. S3
). (C) Stimulation of purified McjD-ATPase by MccJ25 in proteoliposomes. (D) Ligand-stimulated ATPase activity of McjD in detergent solution. (E) Hoechst 33342 transport in proteoliposomes containing an equal amount of McjD or the E506Q mutant. ATP-dependent Hoechst 33342 transport from the membrane into the acidic lumen causes quenching of dye fluorescence for McjD. (F) Hoechst 33342 efflux in intact cells. Inhibition of McjD with ortho-vanadate (Vi) increases Hoechst 33342 accumulation for McjD but not E506Q-McjD. Fluorescence traces are typical for data obtained in three independent experiments using independent batches of cells and proteoliposomes. Error bars are shown for all measurements (mean ± SEM; n = 3); when error bars are not visible, they are contained within the symbols.
Fig. 2.
Structure of the outward occluded conformation of McjD. (A) The McjD structure is viewed in the plane of the membrane. The membrane is shown in gray. The transmembrane helices of one subunit are numbered. Bound AMP-PNP is shown as sticks. (B) The cavity of McjD is shown as gray surface. (C) The electrostatic surface calculation of McjD cavity displays positive and negative charges to bind MccJ25. The cavity is outlined with a broken yellow line. The surfaces are colored from blue (positively charged regions) to red (negatively charged regions). Hydrophobic regions are shown as white. (D) Periplasmic view of the outward occluded McjD (Left) and “winged” outward-open Sav1866 (pdb 2ONJ) (Right) structures show clear differences in helix packing and accessibility to the interior chamber. The NBDs have been omitted for clarity.
Fig. 3.
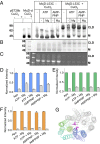
Predictive cross-linking of McjD. The reaction conditions for each lane are indicated above the gels. McjD-L53C was preincubated with appropriate nucleotides for 10 min before cross-linking reactions; cross-linking of McjD-L53C was performed in the presence of 1 mM CuCl2 for 30 min at room temperature. (A) Western-blot analysis of McjD-L53C cross-linked in ISOVs. Nucleotide dependence of intermolecular cysteine cross-linking between the McjD monomers at position 53 is consistent with the formation of an outward-facing occluded state in the ATP-bound state. CLD denotes the formation of the cross-linking dimer and M the monomer in the absence of CuCl2. Leu53Cys mutation is located in the loop that connects TM 1 and 2 that form the occluded structure; the formation of the cross-linking dimer in the presence of nucleotides verifies that the nucleotide-bound occluded state can also exist in the plasma membrane of E. coli. (B) Coomassie-stained SDS/PAGE gel of cross-linked detergent purified McjD. (C) The degree of cross-linking of detergent-purified McjD was estimated in the presence of 7-diethylamino-3-(4'-maleimidylphenyl)-4-methylcoumarin (cpm) that becomes fluorescent upon binding to free cysteines. Formation of the cysteine cross-links resulted in reduced available free cysteines for labeling by the cpm; a small degree of nonspecific binding is observed for the dimeric species by cpm. (D) The relative intensity for each band was estimated by densitometry of the Western blot, (E) Coomassie-stained, and (F) in-gel fluorescence gel; the signal is displayed as fold change in intensity relative to the non–cross-linked reactions. In the absence of CuCl2, the fluorescent signal intensity is high for the monomeric species, M, whereas formation of the CLD has resulted in a very reduced signal. Error bars are shown for all densitometry measurements (mean ± SEM; n = 3). A very small percentage of monomeric species is still present when the cross-linking reactions are terminated. Gray bars represent fraction of monomeric species relative to the total protein present. (G) View from the periplasm of the Leu53 side chain position. Coloring scheme as in Fig. 2_A_. The L53 side chain is shown as magenta stick.
Fig. 4.
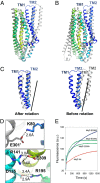
Conformational changes in McjD. The McjD transmembrane helices of one subunit are colored as in Fig. 2_A_. Superposition of McjD with (A) Sav1866 (outward open, gray) and (B) Vibrio cholerae MsbA (inward closed apo, gray). McjD shows structural similarities to the cytoplasmic TM region of Sav1866 and the periplasmic region of MsbA. The most significant conformational changes between McjD and Sav1866 are in TMs 1 and 2. (C) Superposition of TMs 1 and 2 of Sav1866 onto McjD (Left); the Sav1866 TMs 1 and 2 have been rotated around the axis shown (Right). (D) The salt bridges that are stabilizing the occluded conformation are shown as sticks. (Upper) Residues involved in stabilizing TM2 to TM6 (of the opposite monomer, shown in gray). (Lower) Residues that stabilize TM3 to TM6 and TM3 to TM4. The salt-bridge distances are in Å and shown as black broken lines. Color scheme as in Fig. 2_A_. (E) Disruption of the Glu309-R141 salt bridge by E309Q and -A mutations results in reduced ethidium bromide efflux in whole cells compared with wild-type McjD. The transport-inactive E506Q mutant is used as a control.
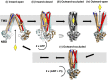
Fig. 5.
Proposed role of the outward-occluded state in the mechanism of ABC exporters. Ligand binding to the inward-open (apo) conformation (state i) facilitates transition of the transporter into the inward-closed (apo) conformation (state ii). ATP binding is associated with a transition into a hypothetical occluded conformation (state iii), and subsequent formation of a nucleotide-bound outward-open conformation (state iv). Upon release of the ligand, the ABC exporter adopts an outward-occluded conformation (state v) (current McjD structure). ATP hydrolysis resets the transporter back to the inward-facing conformation (state i). The TMs 1 and 2 from each monomer are highlighted in dark blue and red. The substrate is shown as a yellow diamond.
Similar articles
- Structural basis for antibacterial peptide self-immunity by the bacterial ABC transporter McjD.
Bountra K, Hagelueken G, Choudhury HG, Corradi V, El Omari K, Wagner A, Mathavan I, Zirah S, Yuan Wahlgren W, Tieleman DP, Schiemann O, Rebuffat S, Beis K. Bountra K, et al. EMBO J. 2017 Oct 16;36(20):3062-3079. doi: 10.15252/embj.201797278. Epub 2017 Sep 1. EMBO J. 2017. PMID: 28864543 Free PMC article. - Conformational Changes of the Antibacterial Peptide ATP Binding Cassette Transporter McjD Revealed by Molecular Dynamics Simulations.
Gu RX, Corradi V, Singh G, Choudhury HG, Beis K, Tieleman DP. Gu RX, et al. Biochemistry. 2015 Sep 29;54(38):5989-98. doi: 10.1021/acs.biochem.5b00753. Epub 2015 Sep 17. Biochemistry. 2015. PMID: 26334959 - Structural Basis for Natural Product Selection and Export by Bacterial ABC Transporters.
Romano M, Fusco G, Choudhury HG, Mehmood S, Robinson CV, Zirah S, Hegemann JD, Lescop E, Marahiel MA, Rebuffat S, De Simone A, Beis K. Romano M, et al. ACS Chem Biol. 2018 Jun 15;13(6):1598-1609. doi: 10.1021/acschembio.8b00226. Epub 2018 May 18. ACS Chem Biol. 2018. PMID: 29757605 - The structure and biological aspects of peptide antibiotic microcin J25.
Vincent PA, Morero RD. Vincent PA, et al. Curr Med Chem. 2009;16(5):538-49. doi: 10.2174/092986709787458461. Curr Med Chem. 2009. PMID: 19199920 Review. - Structural insights into ABC transporter mechanism.
Oldham ML, Davidson AL, Chen J. Oldham ML, et al. Curr Opin Struct Biol. 2008 Dec;18(6):726-33. doi: 10.1016/j.sbi.2008.09.007. Epub 2008 Nov 5. Curr Opin Struct Biol. 2008. PMID: 18948194 Free PMC article. Review.
Cited by
- Structure and mechanism of an active lipid-linked oligosaccharide flippase.
Perez C, Gerber S, Boilevin J, Bucher M, Darbre T, Aebi M, Reymond JL, Locher KP. Perez C, et al. Nature. 2015 Aug 27;524(7566):433-8. doi: 10.1038/nature14953. Epub 2015 Aug 12. Nature. 2015. PMID: 26266984 - Spatial positioning of CFTR's pore-lining residues affirms an asymmetrical contribution of transmembrane segments to the anion permeation pathway.
Gao X, Hwang TC. Gao X, et al. J Gen Physiol. 2016 May;147(5):407-22. doi: 10.1085/jgp.201511557. J Gen Physiol. 2016. PMID: 27114613 Free PMC article. - A single active catalytic site is sufficient to promote transport in P-glycoprotein.
Bársony O, Szalóki G, Türk D, Tarapcsák S, Gutay-Tóth Z, Bacsó Z, Holb IJ, Székvölgyi L, Szabó G, Csanády L, Szakács G, Goda K. Bársony O, et al. Sci Rep. 2016 Apr 27;6:24810. doi: 10.1038/srep24810. Sci Rep. 2016. PMID: 27117502 Free PMC article. - The Nucleotide-Binding Sites of SUR1: A Mechanistic Model.
Vedovato N, Ashcroft FM, Puljung MC. Vedovato N, et al. Biophys J. 2015 Dec 15;109(12):2452-2460. doi: 10.1016/j.bpj.2015.10.026. Biophys J. 2015. PMID: 26682803 Free PMC article. Review. - ABC Transporters in Bacterial Nanomachineries.
Bilsing FL, Anlauf MT, Hachani E, Khosa S, Schmitt L. Bilsing FL, et al. Int J Mol Sci. 2023 Mar 25;24(7):6227. doi: 10.3390/ijms24076227. Int J Mol Sci. 2023. PMID: 37047196 Free PMC article. Review.
References
- Duquesne S, Destoumieux-Garzón D, Peduzzi J, Rebuffat S. Microcins, gene-encoded antibacterial peptides from enterobacteria. Nat Prod Rep. 2007;24(4):708–734. - PubMed
- Rebuffat S, Blond A, Destoumieux-Garzón D, Goulard C, Peduzzi J. Microcin J25, from the macrocyclic to the lasso structure: Implications for biosynthetic, evolutionary and biotechnological perspectives. Curr Protein Pept Sci. 2004;5(5):383–391. - PubMed
- Rosengren KJ, et al. Microcin J25 has a threaded sidechain-to-backbone ring structure and not a head-to-tail cyclized backbone. J Am Chem Soc. 2003;125(41):12464–12474. - PubMed
- Wilson KA, et al. Structure of microcin J25, a peptide inhibitor of bacterial RNA polymerase, is a lassoed tail. J Am Chem Soc. 2003;125(41):12475–12483. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BB/G023425/1/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/H01778X/1/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/I002383/1/Biotechnology and Biological Sciences Research Council/United Kingdom
- WT/099165/Z/12/Z/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources