CNS amyloid-β, soluble APP-α and -β kinetics during BACE inhibition - PubMed (original) (raw)
. 2014 Jun 11;34(24):8336-46.
doi: 10.1523/JNEUROSCI.0540-14.2014.
Maria S Michener 2, Guoxin Wu 3, Bruce W Patterson 4, Robert Chott 4, Vitaliy Ovod 1, Yuriy Pyatkivskyy 1, Kristin R Wildsmith 1, Tom Kasten 1, Parker Mathers 2, Mandy Dancho 2, Christina Lennox 2, Brad E Smith 2, David Gilberto 2, Debra McLoughlin 5, Daniel J Holder 6, Andrew W Stamford 7, Kevin E Yarasheski 4, Matthew E Kennedy 8, Mary J Savage 3, Randall J Bateman 9
Affiliations
- PMID: 24920637
- PMCID: PMC4051982
- DOI: 10.1523/JNEUROSCI.0540-14.2014
CNS amyloid-β, soluble APP-α and -β kinetics during BACE inhibition
Justyna A Dobrowolska et al. J Neurosci. 2014.
Abstract
BACE, a β-secretase, is an attractive potential disease-modifying therapeutic strategy for Alzheimer's disease (AD) as it results directly in the decrease of amyloid precursor protein (APP) processing through the β-secretase pathway and a lowering of CNS amyloid-β (Aβ) levels. The interaction of the β-secretase and α-secretase pathway-mediated processing of APP in the rhesus monkey (nonhuman primate; NHP) CNS is not understood. We hypothesized that CNS inhibition of BACE would result in decreased newly generated Aβ and soluble APPβ (sAPPβ), with increased newly generated sAPPα. A stable isotope labeling kinetics experiment in NHPs was performed with a (13)C6-leucine infusion protocol to evaluate effects of BACE inhibition on CNS APP processing by measuring the kinetics of sAPPα, sAPPβ, and Aβ in CSF. Each NHP received a low, medium, or high dose of MBI-5 (BACE inhibitor) or vehicle in a four-way crossover design. CSF sAPPα, sAPPβ, and Aβ were measured by ELISA and newly incorporated label following immunoprecipitation and liquid chromatography-mass spectrometry. Concentrations, kinetics, and amount of newly generated APP fragments were calculated. sAPPβ and sAPPα kinetics were similar, but both significantly slower than Aβ. BACE inhibition resulted in decreased labeled sAPPβ and Aβ in CSF, without observable changes in labeled CSF sAPPα. ELISA concentrations of sAPPβ and Aβ both decreased and sAPPα increased. sAPPα increased by ELISA, with no difference by labeled sAPPα kinetics indicating increases in product may be due to APP shunting from the β-secretase to the α-secretase pathway. These results provide a quantitative understanding of pharmacodynamic effects of BACE inhibition on NHP CNS, which can inform about target development.
Keywords: BACE inhibitor; SILK; amyloid beta; amyloid precursor protein; sAPPα; sAPPβ.
Copyright © 2014 the authors 0270-6474/14/348336-11$15.00/0.
Figures
Figure 1.
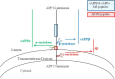
APP and secretase cleavage sites. APP may be cleaved by either β- or α-secretase, and subsequently cleaved by γ-secretase. A concerted β-/γ-secretase cleavage releases Aβ. Indicated on this schematic are the regions of APP where peptides for the SILK study are used to determine labeling of APP metabolites. The sAPPα and sAPPβ peptide (KYLETPGDENEHAHFQ) is in the mid-domain of APP. The Aβ peptide (KLVFFAEDVGSN) is located in the middle of the Aβ sequence. We hypothesize that APP that remains uncleaved by β-secretase due to the presence of a BACE inhibitor will be available for cleavage by α-secretase.
Figure 2.
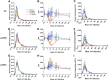
Kinetics of APP metabolites as measured from vehicle-treated NHP CSF (n = 5). A, Averaged 13C6-leucine labeling curve profiles of sAPPα, sAPPβ, and Aβ. B, Maximum MFL for each metabolite was determined. Aβ reached a significantly higher maximum labeling as compared with sAPPα (paired t test, *p = 0.02) and sAPPβ (paired t test, *p = 0.02). C, Extrapolated time of maximum labeling was significantly different among metabolites, with Aβ peaking at t = 14.2 h, while sAPPα and sAPPβ peaked at t = 16.76 h and t = 17.32 h, respectively (Aβ vs. sAPPβ: ***p < 0.0005; Aβ vs. sAPPα: ***p < 0.001; sAPPβ vs. sAPPα: *p = 0.05). D, Mean FSRs indicating fraction-labeled APP metabolites' appearance in the CSF were significantly different from one another (repeated-measures ANOVA, ***p < 0.0001). (Aβ vs. sAPPβ: ***p = 0.0002; Aβ vs. sAPPα: ***p = 0.0006; sAPPβ vs. sAPPα: *p = 0.01) The red dashed line indicates the previously reported NHP FSR of Aβ (10.7%/h; Cook et al., 2010). E, Mean monoexponential slope FCR of Aβ fraction-labeled loss from CSF was significantly higher than the monoexponential slope FCRs of both sAPPα (paired t test, **p = 0.004) and sAPPβ (paired t test, *p = 0.01). There was no statistically significant difference between sAPPα and sAPPβ monoexponential slope FCRs. The red dashed line indicates previously reported NHP FCR of Aβ (9.9%/h; Cook et al., 2010). The black dashed line indicates previously reported human FCR of Aβ (8.3%/h; Bateman et al., 2006). Error bars indicate SD.
Figure 3.
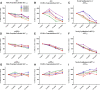
PK of MBI-5 indicate an increase of the BACE inhibitor incorporation into both plasma and CSF with increasing dose. A–C, Individual monkeys' plasma concentrations of MBI-5 after dosing with 10, 30, and 125 mg/kg. D–F, Individual monkeys' CSF concentrations of MBI-5 after dosing with 10, 30, or 125 mg/kg. G, Individual monkeys' plasma AUC59 at each dosage. H, Individual monkeys' CSF AUC59 at each dosage. I, Group-averaged plasma and CSF PK. Error bars indicate SD.
Figure 4.
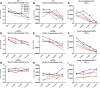
Effects of a BACE inhibitor on SILK relative values, ELISA absolute concentrations, and concentrations of newly generated Aβ (A–C), sAPPβ (D–F), and sAPPα (G–I) in CSF of NHP. A, D, G, SILK MFL Aβ and sAPPβ decreases dose dependently with BACE inhibitor, and MFL sAPPα indicated no measurable difference among vehicle and drug groups (measured by LC-MS). B, E, H, Concentrations of Aβ and sAPPβ decreased dose dependently and absolute concentrations of sAPPα increased dose dependently with a BACE inhibitor (measured by ELISA). C, F, I, Newly generated Aβ and sAPPβ decreased dose dependently and newly generated sAPPα increased dose dependently with a BACE inhibitor (measured as product of LC-MS labeling and ELISA absolute concentrations at each time point). Error bars indicate SD.
Figure 5.
Effects of BACE inhibition on APP metabolites' AUC57. Results are represented as percentage change from vehicle AUC57. Each line represents a particular monkey. A, D, G, MFL Aβ and sAPPβ AUC57 were decreased dose dependently, while MFL sAPPα AUC57 indicated that dosing groups did not significantly differ from the vehicle-treated group. B, E, H, AUC57 values for absolute concentrations of Aβ and sAPPβ were decreased dose dependently, while AUC57 of absolute concentrations of sAPPα presented a dose-dependent increase. C, F, I, AUC57 values for newly synthesized Aβ and sAPPβ were decreased dose dependently, while AUC57 of newly synthesized sAPPα presented a dose-dependent increase.
Figure 6.
Effects of BACE inhibition on APP metabolites' AUC57 without normalization to vehicle group. Each line represents a particular monkey. A, D, G, MFL Aβ and sAPPβ AUC 57 were decreased dose dependently, while MFL sAPPα AUC57 indicated that dosing groups did not significantly differ from the vehicle-treated group. B, E, H, AUC57 values for absolute concentrations of Aβ and sAPPβ were decreased dose dependently, while AUC57 of absolute concentrations of three monkeys' sAPPα increased. Two monkeys did not show significant changes in sAPPα. Coincidentally, these two monkeys had the lowest _C_max for the 125 mg/kg dose among all the monkeys, and were in the lower spectrum for _C_max at the 30 mg/kg dose. C, F, I, AUC57 values for newly synthesized Aβ and sAPPβ were decreased dose dependently, while AUC57 of newly synthesized of sAPPα presented a dose-dependent increase.
Similar articles
- Acute gamma-secretase inhibition of nonhuman primate CNS shifts amyloid precursor protein (APP) metabolism from amyloid-beta production to alternative APP fragments without amyloid-beta rebound.
Cook JJ, Wildsmith KR, Gilberto DB, Holahan MA, Kinney GG, Mathers PD, Michener MS, Price EA, Shearman MS, Simon AJ, Wang JX, Wu G, Yarasheski KE, Bateman RJ. Cook JJ, et al. J Neurosci. 2010 May 12;30(19):6743-50. doi: 10.1523/JNEUROSCI.1381-10.2010. J Neurosci. 2010. PMID: 20463236 Free PMC article. - Heteromers of amyloid precursor protein in cerebrospinal fluid.
Cuchillo-Ibañez I, Lopez-Font I, Boix-Amorós A, Brinkmalm G, Blennow K, Molinuevo JL, Sáez-Valero J. Cuchillo-Ibañez I, et al. Mol Neurodegener. 2015 Jan 8;10:2. doi: 10.1186/1750-1326-10-2. Mol Neurodegener. 2015. PMID: 25573162 Free PMC article. - Soluble amyloid precursor protein α and β in CSF in Alzheimer's disease.
Brinkmalm G, Brinkmalm A, Bourgeois P, Persson R, Hansson O, Portelius E, Mercken M, Andreasson U, Parent S, Lipari F, Ohrfelt A, Bjerke M, Minthon L, Zetterberg H, Blennow K, Nutu M. Brinkmalm G, et al. Brain Res. 2013 Jun 4;1513:117-26. doi: 10.1016/j.brainres.2013.03.019. Epub 2013 Mar 27. Brain Res. 2013. PMID: 23541617 - Guanidine-based β amyloid precursor protein cleavage enzyme 1 (BACE-1) inhibitors for the Alzheimer's disease (AD): A review.
Gehlot P, Kumar S, Kumar Vyas V, Singh Choudhary B, Sharma M, Malik R. Gehlot P, et al. Bioorg Med Chem. 2022 Nov 15;74:117047. doi: 10.1016/j.bmc.2022.117047. Epub 2022 Oct 8. Bioorg Med Chem. 2022. PMID: 36265268 Review. - Functions of Aβ, sAPPα and sAPPβ : similarities and differences.
Chasseigneaux S, Allinquant B. Chasseigneaux S, et al. J Neurochem. 2012 Jan;120 Suppl 1:99-108. doi: 10.1111/j.1471-4159.2011.07584.x. Epub 2011 Dec 7. J Neurochem. 2012. PMID: 22150401 Review.
Cited by
- Insulin resistance in Alzheimer's disease.
Dineley KT, Jahrling JB, Denner L. Dineley KT, et al. Neurobiol Dis. 2014 Dec;72 Pt A:92-103. doi: 10.1016/j.nbd.2014.09.001. Epub 2014 Sep 16. Neurobiol Dis. 2014. PMID: 25237037 Free PMC article. Review. - β-Amyloid peptides and amyloid plaques in Alzheimer's disease.
Gouras GK, Olsson TT, Hansson O. Gouras GK, et al. Neurotherapeutics. 2015 Jan;12(1):3-11. doi: 10.1007/s13311-014-0313-y. Neurotherapeutics. 2015. PMID: 25371168 Free PMC article. Review. - Alzheimer's Disease and Hippocampal Adult Neurogenesis; Exploring Shared Mechanisms.
Hollands C, Bartolotti N, Lazarov O. Hollands C, et al. Front Neurosci. 2016 May 3;10:178. doi: 10.3389/fnins.2016.00178. eCollection 2016. Front Neurosci. 2016. PMID: 27199641 Free PMC article. Review. - Soluble amyloid-β precursor peptide does not regulate GABAB receptor activity.
Rem PD, Sereikaite V, Fernández-Fernández D, Reinartz S, Ulrich D, Fritzius T, Trovo L, Roux S, Chen Z, Rondard P, Pin JP, Schwenk J, Fakler B, Gassmann M, Barkat TR, Strømgaard K, Bettler B. Rem PD, et al. Elife. 2023 Jan 23;12:e82082. doi: 10.7554/eLife.82082. Elife. 2023. PMID: 36688536 Free PMC article. - η-Secretase processing of APP inhibits neuronal activity in the hippocampus.
Willem M, Tahirovic S, Busche MA, Ovsepian SV, Chafai M, Kootar S, Hornburg D, Evans LD, Moore S, Daria A, Hampel H, Müller V, Giudici C, Nuscher B, Wenninger-Weinzierl A, Kremmer E, Heneka MT, Thal DR, Giedraitis V, Lannfelt L, Müller U, Livesey FJ, Meissner F, Herms J, Konnerth A, Marie H, Haass C. Willem M, et al. Nature. 2015 Oct 15;526(7573):443-7. doi: 10.1038/nature14864. Epub 2015 Aug 31. Nature. 2015. PMID: 26322584 Free PMC article.
References
- Alexopoulos P, Tsolakidou A, Roselli F, Arnold A, Grimmer T, Westerteicher C, Leante MR, Förstl H, Livrea P, Kurz A, Perneczky R. Clinical and neurobiological correlates of soluble amyloid precursor proteins in the cerebrospinal fluid. Alzheimers Dement. 2012;8:304–311. doi: 10.1016/j.jalz.2011.04.009. - DOI - PubMed
- Bateman RJ, Siemers ER, Mawuenyega KG, Wen G, Browning KR, Sigurdson WC, Yarasheski KE, Friedrich SW, Demattos RB, May PC, Paul SM, Holtzman DM. A gamma-secretase inhibitor decreases amyloid-β production in the central nervous system. Ann Neurol. 2009;66:48–54. doi: 10.1002/ana.21623. - DOI - PMC - PubMed
- Bernier F, Sato Y, Matijevic M, Desmond H, McGrath S, Burns L, Kaplow JM, Albala B. Clinical study of E2609, a novel BACE1 inhibitor, demonstrates target engagement and inhibition of BACE1 activity in CSF. Alzheimers Dement. 2013;9:P886. doi: 10.1016/j.jalz.2013.08.244. - DOI
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS065667/NS/NINDS NIH HHS/United States
- P30 DK056341/DK/NIDDK NIH HHS/United States
- P41 GM103422/GM/NIGMS NIH HHS/United States
- P60 DK020579/DK/NIDDK NIH HHS/United States
- P30 DK020579/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources