Imaging intraorganellar Ca2+ at subcellular resolution using CEPIA - PubMed (original) (raw)
Imaging intraorganellar Ca2+ at subcellular resolution using CEPIA
Junji Suzuki et al. Nat Commun. 2014.
Free PMC article
Abstract
The endoplasmic reticulum (ER) and mitochondria accumulate Ca(2+) within their lumens to regulate numerous cell functions. However, determining the dynamics of intraorganellar Ca(2+) has proven to be difficult. Here we describe a family of genetically encoded Ca(2+) indicators, named calcium-measuring organelle-entrapped protein indicators (CEPIA), which can be utilized for intraorganellar Ca(2+) imaging. CEPIA, which emit green, red or blue/green fluorescence, are engineered to bind Ca(2+) at intraorganellar Ca(2+) concentrations. They can be targeted to different organelles and may be used alongside other fluorescent molecular markers, expanding the range of cell functions that can be simultaneously analysed. The spatiotemporal resolution of CEPIA makes it possible to resolve Ca(2+) import into individual mitochondria while simultaneously measuring ER and cytosolic Ca(2+). We have used these imaging capabilities to reveal differential Ca(2+) handling in individual mitochondria. CEPIA imaging is a useful new tool to further the understanding of organellar functions.
Figures
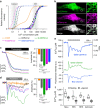
Figure 1. Characterization of CEPIA.
(a) In vitro Ca2+ titration curves of CEPIA1_er_ (black solid), G-CEPIA1_er_ (green solid), R-CEPIA1_er_ (magenta solid), GEM-CEPIA1_er_ (blue solid) compared with cfGCaMP2 (black dotted), G-GECO1.1 (green dotted), R-GECO1 (magenta dotted), GEM-GECO1 (blue dotted) and D1ER (orange). Fitted Hill plot curves are shown. Putative ranges of Ca2+ concentration in the cytosol and ER are indicated (grey boxes). (b) Subcellular distribution of G-CEPIA1_er_ and ER-targeted mCherry (with calreticulin signal sequence) in a HeLa cell. Note that the ER-signal sequence in G-CEPIA1_er_ is different from that of mCherry-er. The images within the white boxes were expanded. Scale bars, 10 μm (left) and 1 μm (right). (c) Comparison of the responses of D1ER, CEPIA1_er_, G-CEPIA1_er_, R-CEPIA1_er_ and GEM-CEPIA1_er_ to thapsigargin (3 μM)-induced depletion of ER Ca2+ in HeLa cells (n_=12–31, mean±s.e.m.; ***P<0.001). Amplitude is defined as the extent of decrease in the fluorescence intensity or ratio after thapsigargin application normalized by the resting value. (d) ER Ca2+ dynamics in response to histamine (10 μM) measured with D1ER, CEPIA1_er, G-CEPIA1_er_, R-CEPIA1_er_ and GEM-CEPIA1_er_ (n_=14–103, mean±s.e.m.; ***P<0.001). Amplitude is defined as the maximum decrease in Δ_F/F_0 or Δ_R/R_0 within a 30-s time window after histamine application. (e) Representative traces of the histamine-induced ER Ca2+ dynamics visualized with GEM-CEPIA1_er in a HeLa cell. Absolute [Ca2+]ER (upper) was estimated from the ratio of the blue to green (blue/green) fluorescence intensities (middle and bottom). (f) Cell type-specific variations of absolute [Ca2+]ER measured with GEM-CEPIA1_er._ Box plots for [Ca2+]ER in a variety of cell types before and after agonist stimulation (10 μM histamine for HeLa cells, 30 μM ATP for HEK cells and cultured astrocytes and 100 nM bradykinin for BHK cells; _n_=4–19) were shown. [Ca2+]ER after agonist stimulation indicated the minimum value reached within 30 s after agonist application. The horizontal line within the box represents the median value, the upper and lower edges of the box represent 75 and 25% values and the whiskers represent the total range.
Figure 2. Wave-like propagation of ER Ca2+ release visualized with G-CEPIA1_er_.
(a) Time-lapse images of wave-like decrease in the ER Ca2+ concentration visualized with G-CEPIA1_er_. Perfusion of 10 μM histamine was started at 0 s. Scale bar, 20 μm. (b) Time course of ER Ca2+ dynamics along the white line in a. (c) Comparison of ER Ca2+ dynamics in two regions of interest in a. The fluorescence intensity was normalized by the initial intensity. Black line: region 1; green line: region 2. (d) The velocity of ER Ca2+ wave measured with G-CEPIA1_er_ (n_=23, mean±s.e.m.) or R-CEPIA1_er (n_=20). For comparison, the velocity of cytosolic Ca2+ wave measured with fluo-4 in cells without (n_=8) or with R-CEPIA1_er expression (Fluo-4+R-CEPIA1_er; _n_=6). There was no significant statistical difference among these values (_P_=0.93, one-way ANOVA).
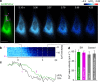
Figure 3. Activity-dependent ER Ca2+ dynamics in cerebellar Purkinje cells visualized with G-CEPIA1_er_.
(a) G-CEPIA1_er_-expressing Purkinje cells in the cerebellar slice. Scale bars, 50 μm (left) and 5 μm (right). (b) PF-induced ER Ca2+ dynamics in the dendrites of Purkinje cells. Time course of mean Δ_F_/F_0 within the white circle (indicated in the left image) indicates fluorescence decrease upon PF inputs (10 stimuli at 100 Hz, grey line). The pseudo-colour image that is the average of 10 consecutive frames (indicated as magenta in the time course of Δ_F/F_0) show local dynamics of luminal Ca2+. Scale bar, 10 μm. (c) PF-induced response in single spine of Purkinje cells. Representative time course of Δ_F/_F_0 within a spine indicated by the arrow. PF inputs (10 stimuli at 100 Hz, gray line) elicited ER Ca2+ release within the spine. Scale bar, 2 μm.
Figure 4. Visualization of ER Ca2+ dynamics during SOCE.
(a,b) Ca2+ dynamics in the ER (lower panels) and cytosol (upper panels) during SOCE. After histamine (10 μM)-induced Ca2+ release in the absence of extracellular Ca2+, SOCE was induced by ‘Ca2+ add back’, the reintroduction of Ca2+ in the extracellular solution (black). To evaluate the contribution of SERCA-dependent Ca2+ uptake by the ER, CPA was applied as indicated to the extracellular solution (a, magenta). The ER Ca2+ refilling was inhibited by Gd3+ (10 μM), an inhibitor of Orai1, during ‘Ca2+ add back’ (b, magenta). (c) Magnified [Ca2+]cyt traces during ‘Ca2+ add back’ in the upper panel of b. (d) Changes in the ER Ca2+ refilling rate and [Ca2+]cyt in response to ‘Ca2+ add back’ with or without Gd3+ application. Left, the slope of linear fitting to the G-CEPIA1_er_ fluorescence change during the intervals _T_1 to _T_3 in b (lower panel) was obtained, and the indicated differences are shown. Right, the average [Ca2+]cyt during the intervals _T_1 to _T_3 in b (upper panel) and c was obtained, and the indicated differences are show. _n_=35 for control and 42 for Gd3+ (mean±s.e.m.). ***P<0.001.
Figure 5. Simultaneous imaging of STIM1 localization and ER Ca2+ concentration.
(a) The images of GEM-CEPIA1_er_ (upper) and mCherry-STIM1 (middle) at three time points indicated in b as grey dotted lines (_T_1, _T_2 and _T_3). The expanded images of mCherry-STIM1 within the white boxes were shown in the lower panels. Scale bars, 20 μm (upper and middle) and 5 μm (lower). (b) Time courses of ER Ca2+ concentration (blue) and the number of mCherry-STIM1 puncta normalized with the minimum and maximum (magenta). As [Ca2+]ER was depleted with histamine stimulation in the Ca2+-free solution, mCherry-STIM1 formed puncta (_T_2). After Ca2+ addback in the external solution, [Ca2+]ER gradually recovered and mCherry-STIM1 puncta disappeared (_T_3). (c) The normalized number of mCherry-STIM1 puncta was plotted against ER Ca2+ concentration during puncta formation (black) and dissociation (magenta). The plots obtained from three independent experiments were overlaid. The relationship between [Ca2+]ER and puncta formation can be fitted by Hill plot with a Hill coefficient of 7.9 and a _K_1**/**2 of 350 μM for puncta formation, and 9.7 and 530 μM for puncta dissociation.
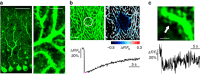
Figure 6. Intercellular heterogeneity of mitochondrial Ca2+ imaging visualized with CEPIA.
(a) Ca2+ titration curves of three mitochondria-targeted CEPIA variants (CEPIA2_mt_, K_d=160 nM, F_max/F_min=1.7; CEPIA3_mt, 11 μM, 1.6; CEPIA4_mt, 56 μM, 1.5). The measurements were performed at pH 8.0. (b) Representative images of HeLa cells expressing CEPIA2_mt (left) co-stained with MitoTracker Red (middle). The merged images are shown in the right panels. The areas within the white boxes were expanded (lower). Scale bars, 10 μm (upper) and 2 μm (lower). (c) Time courses of mitochondrial Ca2+ response using CEPIA2_mt_ in HeLa cells prestimulated with FCCP (magenta) or DMSO (black). Representative trace of 11 cells for vehicle only (DMSO) and 15 cells for FCCP. (d) Representative traces of mitochondrial Ca2+ dynamics upon stimulation with histamine (10 μM) in HeLa cells. To enhance mitochondrial Ca2+ signal, rat MCU was extrinsically expressed (+MCU, blue) or the inhibitor of Na+/Ca2+ exchanger CGP-37157 (10 μM) was applied (+CGP, magenta). (e) The percentage of cells with mitochondrial Ca2+ responses upon histamine application measured with CEPIA2–4_mt_ among control HeLa cells, MCU-expressing cells (+MCU) and CGP-37157 (10 μM)-pretreated cells (+CGP).
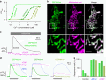
Figure 7. Subcellular heterogeneity of mitochondrial Ca2+ response.
(a) Fluorescence image of a CEPIA3_mt_-expressing HeLa cell. The area within the white box was expanded. Scale bars, 10 μm (left) and 2 μm (right). (b) Time courses of mitochondrial Ca2+ signal during 10 μM histamine application within the two regions of interest shown in a. Time course averaged over the entire cell was also shown (global). Averaged fluorescence images at resting state (T_1, left blue box) and after histamine application (T_2, right blue box) were indicated in c. (c) Upper, averaged fluorescence images at resting state (T_1, left) and after histamine application (T_2, right) as indicated in b. Lower, time-dependent changes in the fluorescence intensity were shown in pseudo-colour. Scale bar, 2 μm. (d) The fluorescence images of a HeLa cell expressing G-CEPIA1_er (green) and mitochondria-localized R-GECO1 (R-GECO1_mt, magenta). The area within the white box was expanded. Scale bars, 5 μm (left) and 1 μm (right). (e) Time courses of Ca2+ signal in the mitochondria, ER and cytosol in a HeLa cell stimulated with 10 μM histamine within the two regions of interest indicated in d. Time courses averaged over the entire cell were also shown (Global). (f) The images of R-GECO1_mt (magenta) and G-CEPIA1_er (green) at three time points were shown. Representative images of six cells. Scale bar, 1 μm.
Similar articles
- Intraorganellar calcium imaging in Arabidopsis seedling roots using the GCaMP variants GCaMP6m and R-CEPIA1er.
Luo J, Chen L, Huang F, Gao P, Zhao H, Wang Y, Han S. Luo J, et al. J Plant Physiol. 2020 Mar-Apr;246-247:153127. doi: 10.1016/j.jplph.2020.153127. Epub 2020 Jan 23. J Plant Physiol. 2020. PMID: 32007728 - Dissection of local Ca(2+) signals inside cytosol by ER-targeted Ca(2+) indicator.
Niwa F, Sakuragi S, Kobayashi A, Takagi S, Oda Y, Bannai H, Mikoshiba K. Niwa F, et al. Biochem Biophys Res Commun. 2016 Oct 7;479(1):67-73. doi: 10.1016/j.bbrc.2016.09.034. Epub 2016 Sep 9. Biochem Biophys Res Commun. 2016. PMID: 27616195 - Imaging of mitochondrial Ca2+ dynamics in astrocytes using cell-specific mitochondria-targeted GCaMP5G/6s: mitochondrial Ca2+ uptake and cytosolic Ca2+ availability via the endoplasmic reticulum store.
Li H, Wang X, Zhang N, Gottipati MK, Parpura V, Ding S. Li H, et al. Cell Calcium. 2014 Dec;56(6):457-66. doi: 10.1016/j.ceca.2014.09.008. Epub 2014 Sep 30. Cell Calcium. 2014. PMID: 25443655 Free PMC article. - Moving Ca2+ from the endoplasmic reticulum to mitochondria: is spatial intimacy enough?
Rutter GA. Rutter GA. Biochem Soc Trans. 2006 Jun;34(Pt 3):351-5. doi: 10.1042/BST0340351. Biochem Soc Trans. 2006. PMID: 16709159 Review. - From Stores to Sinks: Structural Mechanisms of Cytosolic Calcium Regulation.
Enomoto M, Nishikawa T, Siddiqui N, Chung S, Ikura M, Stathopulos PB. Enomoto M, et al. Adv Exp Med Biol. 2017;981:215-251. doi: 10.1007/978-3-319-55858-5_10. Adv Exp Med Biol. 2017. PMID: 29594864 Review.
Cited by
- A bright cyan fluorescence calcium indicator for mitochondrial calcium with minimal interference from physiological pH fluctuations.
Gu W, Yang Y, Wang Y, Li J, Li W, Zhang X, Dong H, Wang Y. Gu W, et al. Biophys Rep. 2024 Oct 31;10(5):315-327. doi: 10.52601/bpr.2024.240001. Biophys Rep. 2024. PMID: 39539283 Free PMC article. - Simultaneous detection of membrane contact dynamics and associated Ca2+ signals by reversible chemogenetic reporters.
García Casas P, Rossini M, Påvénius L, Saeed M, Arnst N, Sonda S, Fernandes T, D'Arsiè I, Bruzzone M, Berno V, Raimondi A, Sassano ML, Naia L, Barbieri E, Sigismund S, Agostinis P, Sturlese M, Niemeyer BA, Brismar H, Ankarcrona M, Gautier A, Pizzo P, Filadi R. García Casas P, et al. Nat Commun. 2024 Nov 12;15(1):9775. doi: 10.1038/s41467-024-52985-0. Nat Commun. 2024. PMID: 39532847 Free PMC article. - Deep-prior ODEs augment fluorescence imaging with chemical sensors.
Pham TA, Boquet-Pujadas A, Mondal S, Unser M, Barbastathis G. Pham TA, et al. Nat Commun. 2024 Oct 24;15(1):9172. doi: 10.1038/s41467-024-53232-2. Nat Commun. 2024. PMID: 39448575 Free PMC article. - Cryo-EM structures of ryanodine receptors and diamide insecticides reveal the mechanisms of selectivity and resistance.
Lin L, Wang C, Wang W, Jiang H, Murayama T, Kobayashi T, Hadiatullah H, Chen YS, Wu S, Wang Y, Korza H, Gu Y, Zhang Y, Du J, Van Petegem F, Yuchi Z. Lin L, et al. Nat Commun. 2024 Oct 20;15(1):9056. doi: 10.1038/s41467-024-53490-0. Nat Commun. 2024. PMID: 39428398 Free PMC article. - Dual role of the S5 segment in type 1 ryanodine receptor channel gating.
Murayama T, Otori Y, Kurebayashi N, Yamazawa T, Oyamada H, Sakurai T, Ogawa H. Murayama T, et al. Commun Biol. 2024 Sep 18;7(1):1108. doi: 10.1038/s42003-024-06787-1. Commun Biol. 2024. PMID: 39294299 Free PMC article.
References
- Brini M. & Carafoli E. Calcium pumps in health and disease. Physiol. Rev. 89, 1341–1378 (2009). - PubMed
- Rizzuto R., De Stefani D., Raffaello A. & Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat. Rev. Mol. Cell Biol. 13, 566–578 (2012). - PubMed
- Berridge M. J., Bootman M. D. & Roderick H. L. Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 4, 517–529 (2003). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous