SETD2-dependent histone H3K36 trimethylation is required for homologous recombination repair and genome stability - PubMed (original) (raw)
SETD2-dependent histone H3K36 trimethylation is required for homologous recombination repair and genome stability
Sophia X Pfister et al. Cell Rep. 2014.
Abstract
Modulating chromatin through histone methylation orchestrates numerous cellular processes. SETD2-dependent trimethylation of histone H3K36 is associated with active transcription. Here, we define a role for H3K36 trimethylation in homologous recombination (HR) repair in human cells. We find that depleting SETD2 generates a mutation signature resembling RAD51 depletion at I-SceI-induced DNA double-strand break (DSB) sites, with significantly increased deletions arising through microhomology-mediated end-joining. We establish a presynaptic role for SETD2 methyltransferase in HR, where it facilitates the recruitment of C-terminal binding protein interacting protein (CtIP) and promotes DSB resection, allowing Replication Protein A (RPA) and RAD51 binding to DNA damage sites. Furthermore, reducing H3K36me3 levels by overexpressing KDM4A/JMJD2A, an oncogene and H3K36me3/2 demethylase, or an H3.3K36M transgene also reduces HR repair events. We propose that error-free HR repair within H3K36me3-decorated transcriptionally active genomic regions promotes cell homeostasis. Moreover, these findings provide insights as to why oncogenic mutations cluster within the H3K36me3 axis.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
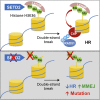
Graphical abstract
Figure 1
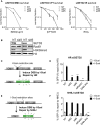
SETD2- and RAD51-Depleted Cells Exhibit a Common Break-Induced Mutation Signature (A) Schematic map of the HPRT+:I-SceI assay, where arrows indicate PCR primers used to amplify genomic DNA to allow sequencing across the break site (see Supplemental Experimental Procedures). (B) Mutation frequency of nontargeting control (NT), SETD2-depleted, RAD51-depleted, and SETD2/RAD51-codepleted cells (siS+siR) after I-SceI induced DSB. Error bars represent SEM and ∗p < 0.05; n.s., not significant. (C) Representative sequence alignments of the above-mentioned PCR products in nontargeting control (NT), SETD2-depleted, RAD51-depleted, and SETD2/RAD51-codepleted backgrounds, respectively. (D) Average length of deletions (bp) in different backgrounds, where each dot represents an independent clone. The lines represent mean and SEM, ∗p < 0.05, ∗∗∗p < 0.001. (E) Frequency of repair by MMEJ in deletion mutants isolated from different backgrounds. p values calculated by statistical analysis “difference between proportions,” ∗p < 0.05. See also Figure S1.
Figure 2
SETD2 Is Required for Homologous Recombination (A) Clonogenic survival of SETD2 knockdown (si#3 and si#5) or nontargeting control (NT) U2OS cells treated with indicated concentrations of MMC, CPT, and IR. Error bars show SEM from three independent experiments. (B) Western blots showing levels of SETD2, RAD51, and H3K36me3 72 hr after siRNA transfection. (C) Schematic map of the DR-GFP cassette for assessing HR efficacy. (D) HR repair efficacy of reporter cells treated with nontargeting control siRNA (NT), SETD2 siRNAs (si#3 and si#5), RAD51 siRNA (siRad51), or DNA-PK inhibitor NU7441 (Axon), indicated by the percentage of GFP-positive cells. Error bars show SEM from three independent experiments. ∗∗∗p < 0.001. (E) Schematic map of the NHEJ cassette. (F) NHEJ repair efficacy of reporter cells treated with nontargeting control siRNA (NT), SETD2 siRNAs (si#3 and si#5), RAD51 siRNA (siRAD51), or DNA-PK inhibitor NU7441, indicated by the percentage of GFP-positive cells. Error bars show SEM from three independent experiments. ∗∗∗p < 0.001. See also Figure S2.
Figure 3
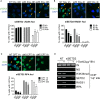
SETD2 Is Required for the Recruitment of RPA and RAD51 (A and B) γH2AX (A) or RAD51 (B) foci formation at indicated times after IR (5 Gy) in U2OS cells treated with nontargeting control siRNA (NT) or SETD2 siRNAs (si#3 and si#5). Error bars represent SEM from three independent experiments. (C) RPA32 foci formation at indicated times after treatment with CPT (10 μM) in U2OS cells transfected with control siRNA (NT) or SETD2 siRNAs (si#3 and si#5). Representative fluorescent images are shown; scale bar, 20 μm. For each condition, 45 fields and at least 500 cells were examined by Incell (GE Healthcare). Error bars show SEM from three independent experiments. ∗∗∗p < 0.001, ∗∗p < 0.01, ∗p < 0.05. (D) ChIP analysis on DR-GFP U2OS cells transfected with nontargeting control (NT) or SETD2 siRNA for 72 hr, followed by transfection of either vector or I-SceI plasmid for a further 18 hr, as indicated. ChIP on the “up” DNA site of the DR-GFP cassette was performed on the lysate using antibodies against nonspecific Ig (NS), H3K36me3 (Abcam), SETD2 (Abcam), RAD51 (Santa Cruz Biotechnology), or RPA (Millipore); inputs (IN) are also indicated. See also Figures S2 and S3.
Figure 4
H3K36me3 Is Required for Efficient HR Repair (A) Schematic map showing site-directed mutagenesis for abolishing the methyltransferase activity of SETD2, with both R and C residues mutated simultaneously. Sequence alignment of human SETD2 and Saccharomyces cerevisiae Set2 and Schizosacchromyces pombe Set2 amino acid sequences reveals evolutionarily conserved residues that reside in the SET domain. (B) RPA32 foci formation at 2 hr after treatment with CPT (10 μM) in cells transfected with SETD2 siRNA. WT: T-REx U2OS clone (see Experimental Procedures) with wild-type SETD2 cDNA integrated but not expressing exogenous SETD2. WT+DOX: the same WT clone expressing exogenous wild-type SETD2. MD: T-REx U2OS clone with methyltransferase-dead mutant SETD2 cDNA integrated but not expressing exogenous SETD2. MD+DOX: the same MD clone expressing exogenous mutant SETD2. Error bars show SEM from three independent experiments, ∗p < 0.05. (C) RAD51 foci formation at 4 hr after IR (5 Gy) in cells transfected with SETD2 siRNA. The clones used are the same as in (B). Error bars show SEM from three independent experiments, ∗∗p < 0.01. (D) HR efficacy in cells transfected with NT or SETD2 siRNA. The clones used are the same as in (B); error bars show SEM from three independent experiments, ∗∗∗p < 0.001. (E) Western blot showing levels of KDM4A, H3K36me3 and H3 following KDM4A induction with 5 μg/ml doxycycline (DOX) for 72 hr in the KDM4A T-REx U2OS cells (see Experimental Procedures). (F) RPA32 foci formation at indicated time after 2 hr CPT (10 μM) treatment in KDM4A T-REx cells treated with or without 5 μg/ml doxycycline (DOX). Error bars show SEM from three independent experiments, ∗p < 0.05. (G) RAD51 foci formation 8 hr after IR (5 Gy) in KDM4A T-REx cells treated with or without 5 μg/ml doxycycline (DOX). Error bars show SEM from three independent experiments. ∗p < 0.05. (H) HR repair efficacy in KDM4A T-REx DR-GFP U2OS cells treated with or without 5 μg/ml doxycycline (DOX). Error bars show SEM for three independent experiments. ∗∗p < 0.01. (I) Western blot showing levels of H3K36me3 and H3 in U2OS cells with stably integrated H3.3 (control) or H3.3K36M lentiviral construct (see Supplemental Experimental Procedures). (J) RPA32 foci formation at indicated time after 2 hr CPT (10 μM) treatment in H3.3 or H3.3K36M stable expression cells. Error bars show SEM from three independent experiments. (K) RAD51 foci formation 8 hr after IR (5 Gy) in H3.3 or H3.3K36M stable expression cells. Error bars show SEM for three independent experiments, ∗p < 0.05. See also Figure S4.
Figure 5
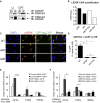
SETD2 Promotes HR through LEDGF/CtIP-Facilitated Resection (A) Coimmunoprecipitation of LEDGF and H3K36me3 in the presence or absence of DNA damage. U2OS cells were left untreated (U) or treated with CPT (15 μM for 4 hr). Reciprocal co-IPs are shown. (B) ChIP analysis of LEDGF binding to the DSB site before and after I-SceI induction in NT or SETD2 knockdown DR-GFP cells. siNT, control siRNA-treated cells before cut; siS, SETD2 siRNA-treated cells before cut; siNT+IScI, control siRNA-treated cells after cut; siS+IScI: SETD2 siRNA-treated cells after cut. Numbers were quantified from one ChIP-PCR experiment and show LEDGF-ChIP over input. (C) Microirradiation showing CtIP recruitment to the damage site in U2OS cells treated with nontargeting control siRNA (NT) or SETD2 siRNAs (si#3 and si#5). Fluorescent images were acquired using a confocal microscope (Zeiss); scale bars, 20 μm. (D) HR efficacy (as measured by GFP reporter assay as in Figure 2D) in cells transfected with control siRNA (NT) or CtIP siRNA (siCtIP) or both CtIP and SETD2 siRNA (siC+siS). Error bars show SEM from three independent experiments, ∗∗p < 0.01, n.s. not significant. (E) CtIP depletion impedes resection at an AsiSI induced DSB. DNA was extracted from 4OHT-treated or untreated DIvA cells, transfected with control or CtIP siRNA, as indicated. Resection was analyzed, using a protocol detailed in Zhou et al. (2014), at an AsiSI-induced DSB reported to be repaired by a RAD51-dependent pathway (DSB-II in Aymard et al., 2014). The mean and SEM (n = 4, technical replicate) of a representative experiment are presented. (F) SETD2 depletion impedes resection at an AsiSI induced DSB. DNA was extracted from 4OHT-treated or untreated DIvA cells, transfected with control or SETD2 siRNA, as indicated. Same as in (E) except that cells were transfected with a siRNA directed against SETD2. The mean and SEM (n = 4, technical replicate) of a representative experiment are presented. See also Figure S5.
Figure 6
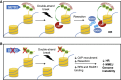
Model for the Role of SETD2-Dependent H3K36me3 in HR and Genome Stability (A) SETD2-dependent H3K36 trimethylation constitutively recruits LEDGF to chromatin. Following DNA double-strand break, LEDGF recruits CtIP, thereby promoting resection, facilitating RPA and RAD51 recruitment. (B) In the absence of H3K36 trimethylation, lack of LEDGF chromatin association results in reduced recruitment of CtIP after double-strand breaks, impairing resection and HR, leading to elevated levels of MMEJ and genome instability.
Similar articles
- The SETD2 Methyltransferase Supports Productive HPV31 Replication through the LEDGF/CtIP/Rad51 Pathway.
Mac M, DeVico BM, Raspanti SM, Moody CA. Mac M, et al. J Virol. 2023 May 31;97(5):e0020123. doi: 10.1128/jvi.00201-23. Epub 2023 May 8. J Virol. 2023. PMID: 37154769 Free PMC article. - SETD2-dependent H3K36me3 plays a critical role in epigenetic regulation of the HPV31 life cycle.
Gautam D, Johnson BA, Mac M, Moody CA. Gautam D, et al. PLoS Pathog. 2018 Oct 12;14(10):e1007367. doi: 10.1371/journal.ppat.1007367. eCollection 2018 Oct. PLoS Pathog. 2018. PMID: 30312361 Free PMC article. - Di- and tri-methylation of histone H3K36 play distinct roles in DNA double-strand break repair.
Chen R, Zhao MJ, Li YM, Liu AH, Wang RX, Mei YC, Chen X, Du HN. Chen R, et al. Sci China Life Sci. 2024 Jun;67(6):1089-1105. doi: 10.1007/s11427-024-2543-9. Epub 2024 Feb 29. Sci China Life Sci. 2024. PMID: 38842635 - Structure and function of the lysine methyltransferase SETD2 in cancer: From histones to cytoskeleton.
Michail C, Rodrigues Lima F, Viguier M, Deshayes F. Michail C, et al. Neoplasia. 2025 Jan;59:101090. doi: 10.1016/j.neo.2024.101090. Epub 2024 Nov 25. Neoplasia. 2025. PMID: 39591760 Free PMC article. Review. - Understanding histone H3 lysine 36 methylation and its deregulation in disease.
Li J, Ahn JH, Wang GG. Li J, et al. Cell Mol Life Sci. 2019 Aug;76(15):2899-2916. doi: 10.1007/s00018-019-03144-y. Epub 2019 May 30. Cell Mol Life Sci. 2019. PMID: 31147750 Free PMC article. Review.
Cited by
- High fidelity of driver chromosomal alterations among primary and metastatic renal cell carcinomas: implications for tumor clonal evolution and treatment.
Kouba EJ, Eble JN, Simper N, Grignon DJ, Wang M, Zhang S, Wang L, Martignoni G, Williamson SR, Brunelli M, Luchini C, Calió A, Cheng L. Kouba EJ, et al. Mod Pathol. 2016 Nov;29(11):1347-1357. doi: 10.1038/modpathol.2016.133. Epub 2016 Jul 29. Mod Pathol. 2016. PMID: 27469331 - Decorating chromatin for enhanced genome editing using CRISPR-Cas9.
Chen E, Lin-Shiao E, Trinidad M, Saffari Doost M, Colognori D, Doudna JA. Chen E, et al. Proc Natl Acad Sci U S A. 2022 Dec 6;119(49):e2204259119. doi: 10.1073/pnas.2204259119. Epub 2022 Dec 2. Proc Natl Acad Sci U S A. 2022. PMID: 36459645 Free PMC article. - Functional Studies on Primary Tubular Epithelial Cells Indicate a Tumor Suppressor Role of SETD2 in Clear Cell Renal Cell Carcinoma.
Li J, Kluiver J, Osinga J, Westers H, van Werkhoven MB, Seelen MA, Sijmons RH, van den Berg A, Kok K. Li J, et al. Neoplasia. 2016 Jun;18(6):339-46. doi: 10.1016/j.neo.2016.04.005. Epub 2016 May 26. Neoplasia. 2016. PMID: 27292023 Free PMC article. - Tackling malignant melanoma epigenetically: histone lysine methylation.
Orouji E, Utikal J. Orouji E, et al. Clin Epigenetics. 2018 Nov 22;10(1):145. doi: 10.1186/s13148-018-0583-z. Clin Epigenetics. 2018. PMID: 30466474 Free PMC article. Review. - Genome Instability Is Promoted by the Chromatin-Binding Protein Spn1 in Saccharomyces cerevisiae.
Thurston AK, Radebaugh CA, Almeida AR, Argueso JL, Stargell LA. Thurston AK, et al. Genetics. 2018 Dec;210(4):1227-1237. doi: 10.1534/genetics.118.301600. Epub 2018 Oct 9. Genetics. 2018. PMID: 30301740 Free PMC article.
References
- Alexandrov L.B., Nik-Zainal S., Wedge D.C., Aparicio S.A., Behjati S., Biankin A.V., Bignell G.R., Bolli N., Borg A., Børresen-Dale A.L., Australian Pancreatic Cancer Genome Initiative. ICGC Breast Cancer Consortium. ICGC MMML-Seq Consortium. ICGC PedBrain Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. - PMC - PubMed
- Arnaudeau C., Lundin C., Helleday T. DNA double-strand breaks associated with replication forks are predominantly repaired by homologous recombination involving an exchange mechanism in mammalian cells. J. Mol. Biol. 2001;307:1235–1245. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- G0500905/MRC_/Medical Research Council/United Kingdom
- C5255/A15935/CRUK_/Cancer Research UK/United Kingdom
- R19583/MRC_/Medical Research Council/United Kingdom
- BB/K019597/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- R066538/MRC_/Medical Research Council/United Kingdom
- 13058/CRUK_/Cancer Research UK/United Kingdom
- BB/H003371/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- G9400953/MRC_/Medical Research Council/United Kingdom
- 300/A13058/CRUK_/Cancer Research UK/United Kingdom
- G1000807/MRC_/Medical Research Council/United Kingdom
- MC_PC_12003/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials