SRT2104 extends survival of male mice on a standard diet and preserves bone and muscle mass - PubMed (original) (raw)
doi: 10.1111/acel.12220. Epub 2014 Jun 16.
Sarah J Mitchell, Alejandro Martin-Montalvo, Robin K Minor, Maria Almeida, Ana P Gomes, Morten Scheibye-Knudsen, Hector H Palacios, Jordan J Licata, Yongqing Zhang, Kevin G Becker, Husam Khraiwesh, José A González-Reyes, José M Villalba, Joseph A Baur, Peter Elliott, Christoph Westphal, George P Vlasuk, James L Ellis, David A Sinclair, Michel Bernier, Rafael de Cabo
Affiliations
- PMID: 24931715
- PMCID: PMC4172519
- DOI: 10.1111/acel.12220
SRT2104 extends survival of male mice on a standard diet and preserves bone and muscle mass
Evi M Mercken et al. Aging Cell. 2014 Oct.
Abstract
Increased expression of SIRT1 extends the lifespan of lower organisms and delays the onset of age-related diseases in mammals. Here, we show that SRT2104, a synthetic small molecule activator of SIRT1, extends both mean and maximal lifespan of mice fed a standard diet. This is accompanied by improvements in health, including enhanced motor coordination, performance, bone mineral density, and insulin sensitivity associated with higher mitochondrial content and decreased inflammation. Short-term SRT2104 treatment preserves bone and muscle mass in an experimental model of atrophy. These results demonstrate it is possible to design a small molecule that can slow aging and delay multiple age-related diseases in mammals, supporting the therapeutic potential of SIRT1 activators in humans.
Keywords: healthspan; inflammation; lifespan; muscle wasting; osteoporosis; sirtuins.
Published 2014. This article is a U.S. Government work and is in the public domain in the USA.
Figures
Figure 1
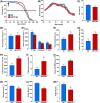
SRT2104 treatment improves whole-body physiology and extends lifespan in mice fed a standard diet. (A) Kaplan–Meier survival curves of mice fed a standard diet (SD) or a SD supplemented with SRT2104. The arrow at 28 weeks indicates the age at which SRT2104 treatment was started. (B–M) The following parameters were analyzed in SD-fed mice without and with SRT2104 supplementation: (B) bodyweights; (C) percentage fat mass; (D) average caloric intake; (E) spontaneous locomotor activity; (F) treadmill performance; (G) time to fall from an accelerating rotarod; (H) trabecular bone volume; (I) trabecular connectivity; (J) trabecular bone mineral density (BMD); (K) circulating glucose and (L) insulin levels were measured after 16 h of fasting; (M) homeostatic measure of insulin resistance (HOMA-IR) index. Data are shown as mean ± SEM. *P < 0.05 compared with SD-fed animals. BV, bone volume; TV, total volume; Tb, trabecular.
Figure 2
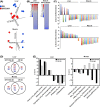
SRT2104 changes the gene expression profile differently in liver and muscle. (A) Principal component analysis (PCA) was performed on liver and muscles tissues of mice fed a SD or SD supplemented with SRT2104. (B) Parametric analysis of gene-set enrichment (PAGE) analysis was performed on microarray data from mice fed a SD or subjected to SRT2104. Columns show pathways significantly upregulated (red) or downregulated (blue) by SRT2104 treatment. See also Tables S5–S7. (C) Effect of SRT2104 on inflammatory and mitochondrial-related pathways from the PAGE analysis for liver and skeletal muscle. (D) Venn diagrams of overlapping gene sets significantly modified by SRT2104 vs. calorie restriction (CR). Upregulated gene sets are depicted in red and the downregulated gene sets in blue. (E) Effect of SRT2104 and CR on select gene sets from mouse liver and muscle. The list of the significantly modified gene sets can be found in Tables S6 and S7. SD, standard diet.
Figure 3
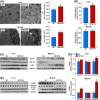
SRT2104 treatment increases mitochondrial content and suppresses the inflammatory response. (A) Representative transmission electron micrographs of liver and muscle, and the respective mitochondrial quantification. (B) Citrate synthase activity. (C) Representative immunoblots from inflammatory markers in liver and muscle tissues. (D) mRNA levels of COMMD genes assessed by quantitative real-time PCR. Relative expression values were normalized to SD-fed mice. (E) Representative immunoblots from oxidative stress markers in liver and muscles. Data are shown as mean ± SEM. *P < 0.05 compared with SD-fed mice.
Figure 4
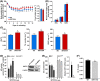
Short-term SRT2104 treatment preserves muscle and bone mass. (A) Bodyweights during 14 days of hindlimb suspension and average food consumption (inset) for 6-month-old mice fed either a standard diet (SD) or SD supplemented with SRT2104 for 6 weeks. (B) Muscle weights. (C) Trabecular bone volume, trabecular connectivity, and trabecular bone mineral density (BMD). (D) Alkaline phosphatase (AP) activity in C2C12 cells infected with SIRT1 shRNA or nontargeting shRNA control and treated with 1 and 3 μ
m
SRT2104 for 24 h. (E) Osteoclast (OC) number in bone marrow-derived osteoblastic cells from wild-type (WT) mice and SIRT1f/f mice and treated with 1 and 3 μ
m
SRT2104 for 4 days. (F) Cortical thickness in femurs from wild-type (WT) and SIRT1KO mice. Data are mean ± SEM. *P < 0.05. SOL, soleus; PL, plantaris; GAS, gastrocnemius; BV, bone volume; TV, total volume; Tb, trabecular.
Similar articles
- SRT1720 improves survival and healthspan of obese mice.
Minor RK, Baur JA, Gomes AP, Ward TM, Csiszar A, Mercken EM, Abdelmohsen K, Shin YK, Canto C, Scheibye-Knudsen M, Krawczyk M, Irusta PM, Martín-Montalvo A, Hubbard BP, Zhang Y, Lehrmann E, White AA, Price NL, Swindell WR, Pearson KJ, Becker KG, Bohr VA, Gorospe M, Egan JM, Talan MI, Auwerx J, Westphal CH, Ellis JL, Ungvari Z, Vlasuk GP, Elliott PJ, Sinclair DA, de Cabo R. Minor RK, et al. Sci Rep. 2011;1:70. doi: 10.1038/srep00070. Epub 2011 Aug 18. Sci Rep. 2011. PMID: 22355589 Free PMC article. - P53/NRF2 mediates SIRT1's protective effect on diabetic nephropathy.
Ma F, Wu J, Jiang Z, Huang W, Jia Y, Sun W, Wu H. Ma F, et al. Biochim Biophys Acta Mol Cell Res. 2019 Aug;1866(8):1272-1281. doi: 10.1016/j.bbamcr.2019.04.006. Epub 2019 Apr 6. Biochim Biophys Acta Mol Cell Res. 2019. PMID: 30959066 - Axonal protection by a small molecule SIRT1 activator, SRT2104, with alteration of autophagy in TNF-induced optic nerve degeneration.
Kitaoka Y, Sase K, Tsukahara C, Fujita N, Tokuda N, Kogo J, Takagi H. Kitaoka Y, et al. Jpn J Ophthalmol. 2020 May;64(3):298-303. doi: 10.1007/s10384-020-00731-6. Epub 2020 Mar 10. Jpn J Ophthalmol. 2020. PMID: 32157485 - Emerging roles of SIRT1 activator, SRT2104, in disease treatment.
Chang N, Li J, Lin S, Zhang J, Zeng W, Ma G, Wang Y. Chang N, et al. Sci Rep. 2024 Mar 6;14(1):5521. doi: 10.1038/s41598-024-55923-8. Sci Rep. 2024. PMID: 38448466 Free PMC article. Review. - Are sirtuins viable targets for improving healthspan and lifespan?
Baur JA, Ungvari Z, Minor RK, Le Couteur DG, de Cabo R. Baur JA, et al. Nat Rev Drug Discov. 2012 Jun 1;11(6):443-61. doi: 10.1038/nrd3738. Nat Rev Drug Discov. 2012. PMID: 22653216 Free PMC article. Review.
Cited by
- Cerebral Perfusion Enhancing Interventions: A New Strategy for the Prevention of Alzheimer Dementia.
de la Torre JC. de la Torre JC. Brain Pathol. 2016 Sep;26(5):618-31. doi: 10.1111/bpa.12405. Brain Pathol. 2016. PMID: 27324946 Free PMC article. Review. - Organic and Peptidyl Constituents of Snake Venoms: The Picture Is Vastly More Complex Than We Imagined.
Villar-Briones A, Aird SD. Villar-Briones A, et al. Toxins (Basel). 2018 Sep 26;10(10):392. doi: 10.3390/toxins10100392. Toxins (Basel). 2018. PMID: 30261630 Free PMC article. - The Role of Sirtuins in Sarcopenia and Frailty.
Anwar M, Pradhan R, Dey S, Kumar R. Anwar M, et al. Aging Dis. 2023 Feb 1;14(1):25-32. doi: 10.14336/AD.2022.0622. eCollection 2023 Feb 1. Aging Dis. 2023. PMID: 36818553 Free PMC article. - Frailty index as a biomarker of lifespan and healthspan: Focus on pharmacological interventions.
Palliyaguru DL, Moats JM, Di Germanio C, Bernier M, de Cabo R. Palliyaguru DL, et al. Mech Ageing Dev. 2019 Jun;180:42-48. doi: 10.1016/j.mad.2019.03.005. Epub 2019 Mar 26. Mech Ageing Dev. 2019. PMID: 30926563 Free PMC article. Review. - Mesenchymal Stem Cell Senescence and Osteogenesis.
Tjempakasari A, Suroto H, Santoso D. Tjempakasari A, et al. Medicina (Kaunas). 2021 Dec 31;58(1):61. doi: 10.3390/medicina58010061. Medicina (Kaunas). 2021. PMID: 35056369 Free PMC article. Review.
References
- Barger JL, Kayo T, Vann JM, Arias EB, Wang J, Hacker TA, Wang Y, Raederstorff D, Morrow JD, Leeuwenburgh C, Allison DB, Saupe KW, Cartee GD, Weindruch R, Prolla TA. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS ONE. 2008;3:e2264. - PMC - PubMed
- Baur J, Pearson K, Price N, Jamieson H, Lerin C, Kalra A, Prabhu V, Allard J, Lopez-Lluch G, Lewis K, Pistell P, Poosala S, Becker K, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein K, Spencer R, Lakatta E, Le Couteur D, Shaw R, Navas P, Pueigserver P, Ingram K, de Cabo R, Sinclair D. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. - PMC - PubMed
- Bordone L, Cohen D, Robinson A, Motta MC, Van Veen E, Czopik A, Steele AD, Crowe H, Marmor S, Luo J, Gu W, Guarente L. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007;6:759–767. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 AG028730/AG/NIA NIH HHS/United States
- Z01 AG000363-01/Intramural NIH HHS/United States
- ZIA AG000363-06/Intramural NIH HHS/United States
- R01AG028730/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases