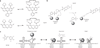Advances in fluorescence labeling strategies for dynamic cellular imaging - PubMed (original) (raw)
Review
Advances in fluorescence labeling strategies for dynamic cellular imaging
Kevin M Dean et al. Nat Chem Biol. 2014 Jul.
Abstract
Synergistic advances in optical physics, probe design, molecular biology, labeling techniques and computational analysis have propelled fluorescence imaging into new realms of spatiotemporal resolution and sensitivity. This review aims to discuss advances in fluorescent probes and live-cell labeling strategies, two areas that remain pivotal for future advances in imaging technology. Fluorescent protein- and bio-orthogonal-based methods for protein and RNA imaging are discussed as well as emerging bioengineering techniques that enable their expression at specific genomic loci (for example, CRISPR and TALENs). Important attributes that contribute to the success of each technique are emphasized, providing a guideline for future advances in dynamic live-cell imaging.
Figures
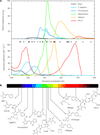
Figure 1. Spectral properties of chromophore classes found in FPs
(a) Top, fluorescence emission spectra represented in terms of brightness (ε × ϕFl), normalized to the brightest FP available (mNeonGreen). Bottom, absorption spectra of select FPs. Minor dots represent a cross section of solid-state laser sources that are commercially available and major dots are those typically equipped on microscopy equipment. Colors approximate the emission wavelength of the FP. (b) Chromophores and cofactors responsible for fluorescence in select constitutive FPs shown.
Figure 2. Covalent bio-orthogonal labeling mechanisms
Each panel provides the approximate size of the fusion protein, shown as a crystal structure. Chemical reactions have been enlarged for clarity, and all of the rates are measured in M−1 s−1. For all panels, scale bar (at bottom) represents 1.5 nm. (a) Genetic fusion of SNAP-tag to a protein of interest and subsequent suicide inhibition with fluorescent O6-benzylguanine derivatives results in a fluorescently labeled protein (Protein Data Bank (PDB) code 3KYZ). The fluorophore shown is SiR-SNAP, a silicon-based near-infrared fluorophore. (b) In the same way as SNAP-tag, CLIP-tag reacts with fluorescent O2-benzylcytosine derivatives (PDB code 3KYZ). (c) HaloTag fusion proteins react with chlorinated haloalkanes to form an irreversible fluorophore-alkyl-enzyme tether (PDB code 4KAA). (d) TMP-tag binds trimethoprim with nanomolar affinity and accelerates thiol reactivity with an acrylamide appendage (right) through proximity effects, irreversibly labeling the protein of interest (PDB code 1RD7). (e) Lipoic acid ligase, expressed in trans to the protein of interest labeled with a 13-amino-acid peptide, catalyzes the site-specific incorporation of coumarin. (f) Biotin ligase, which is analogous to lipoic acid ligase, catalyzes the site-specific incorporation of biotin or biotin isomeres, which can be chemically modified or used as an antigen for subsequent detection.
Figure 3. Bio-orthogonal labeling mechanisms based on reversible binding equilibria
(a) FlAsH (top) and ReAsH (bottom) recognize a specific peptide motif (Cys-Cys-Pro-Gly-Cys-Cys) with high affinity (_K_D ~4 pM) and undergo a large increase in fluorescence intensity upon binding. (b) Ligand-specific binding by a protein, RNA or generic chelating agent triggers proximity-induced reactivity between a nucleophile located on the chelating group and the reactive tosyl-conjugated fluorophore. (c) A multidomain RNA construct with a target-specific aptamer domain and a target-dependent ′Spinach′ domain. An endogenous protein serves as the ligand for the aptamer domain, and binding of the protein triggers a conformational rearrangement in the Spinach domain, allowing productive binding of DFHBI to yield a fluorescent state. In the absence of the antigen, Spinach cannot bind DFHBI and remains nonfluorescent.
Figure 4. Methods for labeling RNA biomolecules
(a,b) Initial efforts to label RNAs in vivo were performed on chemically fixed samples and included long antisense DNA and RNA oligonucleotides labeled with a single fluorophore (shown as a star) (a) or multiple shorter oligonucleotides, each labeled with a single fluorophore (b). (c) Branched DNA structures provide increased specificity through the use of two adaptor oligonucleotides that nucleate the formation of a branched and multiply labeled oligonucleotides analogous to DNA origami. (d) A ‘molecular beacon’ fluorophore-quencher conjugate remains nonfluorescent until hybridization with the sequence of interest. (e) For live-cell imaging, 24 tandem repeats of a hairpin structure are inserted into the 3′ untranslated region of a transcript. Two GFP-MS2 (or equivalent orthogonal phage protein) fusion proteins identify each hairpin motif and bind with high affinity, generating a transcript with 48 GFP molecules and enabling routine single-molecule imaging of RNA in live cells. (f) To eliminate nonspecific background, a multicomponent system (for example, Pum-HD) oligomerizes on a unique RNA hairpin structure, triggering bimolecular complementation of GFP. (g) DFHBI, which is a GFP chromophore analog that is nonfluorescent when free in solution, is recognized by a specific RNA aptamer sequence, ′Spinach′, that turns on fluorescence upon binding.
Similar articles
- Recent progress in design of protein-based fluorescent biosensors and their cellular applications.
Tamura T, Hamachi I. Tamura T, et al. ACS Chem Biol. 2014 Dec 19;9(12):2708-17. doi: 10.1021/cb500661v. Epub 2014 Oct 23. ACS Chem Biol. 2014. PMID: 25317665 Review. - Dark dyes-bright complexes: fluorogenic protein labeling.
Bruchez MP. Bruchez MP. Curr Opin Chem Biol. 2015 Aug;27:18-23. doi: 10.1016/j.cbpa.2015.05.014. Epub 2015 Jun 6. Curr Opin Chem Biol. 2015. PMID: 26056741 Free PMC article. Review. - Structural biology: How fluorescent RNA gets its glow.
Scott WG. Scott WG. Nature. 2014 Sep 4;513(7516):42-3. doi: 10.1038/513042a. Nature. 2014. PMID: 25186897 No abstract available. - Multicolor protein labeling in living cells using mutant β-lactamase-tag technology.
Watanabe S, Mizukami S, Hori Y, Kikuchi K. Watanabe S, et al. Bioconjug Chem. 2010 Dec 15;21(12):2320-6. doi: 10.1021/bc100333k. Epub 2010 Oct 20. Bioconjug Chem. 2010. PMID: 20961132 - Monitoring of RNA Dynamics in Living Cells Using PUM-HD and Fluorescent Protein Reconstitution Technique.
Yoshimura H, Ozawa T. Yoshimura H, et al. Methods Enzymol. 2016;572:65-85. doi: 10.1016/bs.mie.2016.03.018. Epub 2016 Apr 12. Methods Enzymol. 2016. PMID: 27241750
Cited by
- Abstracting the principles of development using imaging and modeling.
Xiong F, Megason SG. Xiong F, et al. Integr Biol (Camb). 2015 Jun;7(6):633-42. doi: 10.1039/c5ib00025d. Integr Biol (Camb). 2015. PMID: 25946995 Free PMC article. Review. - Fe3+-Sensitive Carbon Dots for Detection of Fe3+ in Aqueous Solution and Intracellular Imaging of Fe3+ Inside Fungal Cells.
Chen Y, Sun X, Pan W, Yu G, Wang J. Chen Y, et al. Front Chem. 2020 Jan 15;7:911. doi: 10.3389/fchem.2019.00911. eCollection 2019. Front Chem. 2020. PMID: 32010664 Free PMC article. - Selective Mitochondrial Protein Labeling Enabled by Biocompatible Photocatalytic Reactions inside Live Cells.
Wang H, Zhang Y, Zeng K, Qiang J, Cao Y, Li Y, Fang Y, Zhang Y, Chen Y. Wang H, et al. JACS Au. 2021 Jun 14;1(7):1066-1075. doi: 10.1021/jacsau.1c00172. eCollection 2021 Jul 26. JACS Au. 2021. PMID: 34467350 Free PMC article. - Bioisostere-conjugated fluorescent probes for live-cell protein imaging without non-specific organelle accumulation.
Kamikawa T, Hashimoto A, Yamazaki N, Adachi J, Matsushima A, Kikuchi K, Hori Y. Kamikawa T, et al. Chem Sci. 2024 May 1;15(21):8097-8105. doi: 10.1039/d3sc06957e. eCollection 2024 May 29. Chem Sci. 2024. PMID: 38817570 Free PMC article. - Characterization of flavin binding in oxygen-independent fluorescent reporters.
Anderson NT, Weyant KB, Mukherjee A. Anderson NT, et al. AIChE J. 2020 Dec;66(12):e17083. doi: 10.1002/aic.17083. Epub 2020 Oct 2. AIChE J. 2020. PMID: 34305141 Free PMC article.
References
- Kanchanawong P, et al. Nanoscale architecture of integrin-based cell adhesions. Nature. 2010;468:580–584. An elegant study on the three-dimensional nanostructure of mechanochemical signaling domains using interferometry-based super-resolution imaging.
- Toettcher JE, Weiner OD, Lim WA. Using optogenetics to interrogate the dynamic control of signal transmission by the Ras/Erk module. Cell. 2013;155:1422–1434. This article demonstrates the power of fluorescence imaging coupled with optogenetics and proteomics for interrogation of frequency-dependent signal transduction.
- Boch J, et al. Breaking the code of DNA binding specificity of TAL-type III effectors. Science. 2009;326:1509–1512. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources