Purinergic P2Y₁₄ receptor modulates stress-induced hematopoietic stem/progenitor cell senescence - PubMed (original) (raw)
Purinergic P2Y₁₄ receptor modulates stress-induced hematopoietic stem/progenitor cell senescence
Joonseok Cho et al. J Clin Invest. 2014 Jul.
Abstract
Purinergic receptors of the P2Y family are G protein-coupled surface receptors that respond to extracellular nucleotides and can mediate responses to local cell damage. P2Y-dependent signaling contributes to thrombotic and/or inflammatory consequences of tissue injury by altering platelet and endothelial activation and immune cell phagocytosis. Here, we have demonstrated that P2Y14 modifies cell senescence and cell death in response to tissue stress, thereby enabling preservation of hematopoietic stem/progenitor cell function. In mice, P2Y14 deficiency had no demonstrable effect under homeostatic conditions; however, radiation stress, aging, sequential exposure to chemotherapy, and serial bone marrow transplantation increased senescence in animals lacking P2Y14. Enhanced senescence coincided with increased ROS, elevated p16(INK4a) expression, and hypophosphorylated Rb and was inhibited by treatment with a ROS scavenger or inhibition of p38/MAPK and JNK. Treatment of WT cells with pertussis toxin recapitulated the P2Y14 phenotype, suggesting that P2Y14 mediates antisenescence effects through Gi/o protein-dependent pathways. Primitive hematopoietic cells lacking P2Y14 were compromised in their ability to restore hematopoiesis in irradiated mice. Together, these data indicate that P2Y14 on stem/progenitor cells of the hematopoietic system inhibits cell senescence by monitoring and responding to the extracellular manifestations of tissue stress and suggest that P2Y14-mediated responses prevent the premature decline of regenerative capacity after injury.
Figures
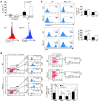
Figure 1. P2Y14 deficiency increases the susceptibility of HSPCs to radiation stress.
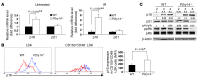
(A) Q-PCR analysis of P2ry14 mRNA: mRNA from BM cells bearing the indicated phenotype was analyzed by Q-PCR. The expression was normalized to GAPDH. The expression level in lineage positive (Lin+) cells was arbitrarily set to 1. Q-PCR was done in duplicate. B, B cells (B220+); T, T cells (CD3+); mono, monocytes (CD11b+). (B) Cells were gated as indicated, and the expression of P2Y14 was measured within the gates. The percentage of P2Y14-expressing (P2Y14+) cells in indicated compartments is plotted on the y axis. The data are representative of at least 3 independent experiments, each with 3 mice per group. (C) Mice of the indicated genotypes were exposed to TBI (3 × 5 Gy). Recipients were allowed to recover for 15 days before the next dose was administered. The number of BM cells was counted within the marrow of femur and tibia. (D) The number of LSK cells was measured after TBI (3 × 5 Gy TBI, 15 days apart). Data show representative mice of at least 6 animals analyzed per group. Statistical analyses were carried out using 1-tailed Student’s t test (C and D) and 2-tailed Student’s t test (B). *P < 0.05; **P < 0.01.
Figure 2. P2Y14 deficiency increases the susceptibility of HSPCs to radiation-induced senescence and cell death.
(A) Mice of the indicated genotypes were exposed to TBI (6 Gy). SA–β-gal activity was determined using C12FDG. Percentage of SA–β-gal–positive LSK population (upper) is expressed as mean ± SD. The data are representative of 6 mice per group. Representative histograms of SA–β-gal staining in gated LSK cell (lower). (B) BM cells from irradiated P2ry14–/– and WT mice (6 Gy TBI) were stained with the indicated antibodies. Lineage-committed progenitor cells were gated based on defined phenotypic criteria, and cellular senescence was measured in each gated population. Numbers indicate the percentage of C12FDG+ cells in the indicated gates. The accompanying graph shows the mean percentage of C12FDG+ cells (± SD) in each gated population (n = 5/genotype). (C) Cell death analysis in gated LSK cells. Cell death was measured by quantification of annexin V+ or DAPI+ (gated on annexin V–) cells 8 hours after TBI (6 Gy). The data are representative of 2 independent experiments each, with BM cells pooled from 2 mice per group. NAC (100 mg/kg) was injected s.c. 4 hours before and 2 hours after TBI. Representative flow cytometric analysis of annexin V+ (upper left) and DAPI+, annexin V– (lower left) LSK cells is shown. Percentages of gated cell populations are indicated. Statistical analyses were carried out using 1-tailed Student’s t test (C) and 2-tailed Student’s t test (A and B). *P < 0.05; **P < 0.01.
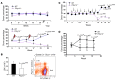
Figure 3. P2Y14 deficiency increases the susceptibility of HSPCs to various hematological stresses.
(A) A mixture of BM cells from P2ry14–/– (CD45.2) and congenic WT (CD45.1) mice were transplanted into recipients (CD45.1.2, n = 16) as described in Methods. The x axis denotes the number of weeks after transplantation. Upper panel: untreated mice (n = 6); lower panel; 5 weeks after transplantation, a group of recipients (n = 10) were irradiated (6 Gy TBI) and then the contributions of WT (CD45.1) and P2ry14–/– (CD45.2) cells to the recipients’ blood were measured. Lightning bolts mark the timing of IR. (B) BM cells from P2ry14–/– and WT mice were transplanted as described in A. Eight to nine months after transplantation, recipients (n = 8) were irradiated (6 Gy) and the frequency of WT and P2ry14–/– derived LSK cells in recipients’ BM was assessed at 5 weeks after IR (right). The accompanying graph (left) shows the absolute numbers of LSK cells. (C) WT and P2ry14–/– BM cells were transplanted as described in A. Five weeks after transplantation, recipients (n = 6) were treated with 5-FU (150 mg/kg). Recipients were allowed to recover for 9 days before the next dose was administered. The x axis denotes the number of weeks after transplantation. Arrows mark the timing of injection. Ratio of WT and P2ry14–/– cells in recipients’ blood was determined 7 days after each 5-FU injection. (D) Contribution of WT and P2ry14–/– cells after secondary transplantation (n = 4/genotype). The x axis denotes the number of weeks after transplantation. Statistical analyses were carried out using 1-tailed Student’s t test (B) and 2-tailed Student’s t test (A, C, and D). *P < 0.05; **P < 0.01; ***P < 0.001.
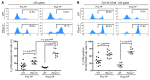
Figure 4. Impact of P2Y14 deficiency on HSPC senescence during the natural aging process.
(A and B) KO and WT mice were maintained in a pathogen-free environment for 90–100 weeks before collection of BM cells for senescence analysis. Representative flow cytometric analyses of C12FDG in LSK (A) and CD150+CD48– LSK (B) cells from WT and P2ry14–/– mice are shown. Percentages of gated cell populations are indicated. BM cells isolated from 90- to 100-week-old WT and KO mice (CD45.2) were further transplanted into lethally irradiated recipient mice (CD45.1.2). Donor-derived CD45.2+ LSK (A, Post-TP) and CD45.2+CD150+CD48– LSK (B, Post-TP) cells were analyzed for SA–β-gal–positive cells using C12FDG. The accompanying graphs show the mean percentage of C12FDG-positive LSK (A) and CD150+CD48– LSK (B) cells. The 2-tailed Student’s _t_-test was used. Pre-TP, pretransplantation; post-TP, post-transplantation. ***P < 0.001.
Figure 5. Impact of P2Y14 deficiency on radiation-induced senescence during embryonic development.
(A and B) HT mice were mated, and pregnant female mice were either untreated or treated (IR) with single TBI at a dose of 1.5 Gy on day 11.5 of gestation. The yolk sac was dissected and used as a source of DNA for genotyping. (A) E18.5 embryos isolated from untreated (upper) and treated (1.5 Gy TBI, lower) pregnant dams were weighed. (B) Representative images of SA–β-gal stained embryos are shown. Numbers below the bottom panel denote percentage of SA–β-gal positive embryos for each indicated genotype. Numbers in parentheses indicate the number of positively stained embryos**/**total number of embryo stained. (C) MEFs were prepared from E12.5–E13.5 P2ry14–/– and WT embryos. Cell numbers were determined at each passage prior to redilution. The 2-tailed Student’s t test was used. (D) SA–β-gal staining of WT and P2ry14–/– MEF cells: MEF cells at passage 4 were subjected to SA–β-gal staining (left). The number of SA–β-gal–positive cells was counted, and the percentage of SA– β-gal positive cells is shown on the y axis (right). At least 50 cells from 3 random fields were counted. The 2-tailed Student’s t test was used. Note that P2ry14–/– MEFs developed a senescence-like morphology, such as a large and flattened morphology (left). Data are representative of 4 independent experiments. Scale bars: 50 μm (left panels); 20 μm (right panels). *P < 0.05.
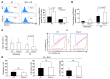
Figure 6. P2Y14 is involved in the modulation of cellular redox homeostasis.
(A and B) MitoSOX staining in gated LSK cells. Mitochondrial superoxide was measured within LSK cells in vivo (6 Gy TBI) (A) and in vitro (1.6 Gy, in vitro IR) (B). NAC was treated as described in the legend to Figure 2. Representative histograms of MitoSOX staining in gated LSK cell (A, left). The accompanying graphs show percentage of LSK cells positive for MitoSOX fluorescence in vivo (A) and in vitro (B). The data are representative of at least 3 independent experiments. The 1-tailed Student’s t test was used. (C) JC-1 staining in gated LSK cells. WT and P2ry14–/– BM cells were irradiated as described in B. A decrease in the ratio of red (FL2: 585 nm) to green (FL1: 530 nm) indicates mitochondrial depolarization. The data are representative of 3 independent experiments, each with BM cells pooled from at least 2 mice per group. The 1-tailed Student’s t test was used. (D) Mice of the indicated genotypes were exposed to TBI (3 × 5 Gy, 15 days apart). NAC (100 mg/kg) was injected s.c. 4 hours before and 2 hours after TBI and once daily thereafter for 6 days. This procedure was repeated after each TBI. The number of total BM (left) and LSK (middle) cells was counted. Mice (n = 4) were individually analyzed for each group. Right: mice (n = 6/genotype) were exposed to TBI (6 Gy). NAC was administered as described in Figure 2C, and percentage of SA–β-gal+ LSK cells was assessed. *P < 0.05; **P < 0.01.
Figure 7. Impact of P2Y14 deficiency on senescence-associated molecules.
(A) WT and P2ry14–/– BM cells were transplanted as described. Eight months after transplantation, recipient mice were either left untreated (n = 4, left) or irradiated (n = 4, right) with TBI (6 Gy). LSK cells derived from WT (CD45.1+) or P2ry14–/– (CD45.2+) donors in the recipients were sorted and subjected to Q-PCR analysis, respectively. The expression level in WT cells was arbitrarily set to 1. The fold change in expression of each gene was calculated using the ΔΔCt method. The expression was normalized to GAPDH. The 2-tailed Student’s t test was used. (B) Four weeks after TBI (6 Gy), LSK and CD150+CD48– LSK cells were gated and analyzed for the expression of p16Ink4a by flow cytometry analysis. Mice were analyzed individually (n > 3 mice/group). Representative flow cytometric analysis of p16Ink4a in gated LSK and CD150+CD48–LSK cells is shown (left). The 2-tailed Student’s _t_-test was used. (C) Western blot analysis of WT and P2ry14–/– MEF cells: early passage WT or P2ry14–/– MEFs were prepared and serially passaged following a 3T3 protocol. Cell lysates were probed with the indicated antibodies. Autoradiographs were analyzed by densitometry. The intensity observed in passage no. 2 WT MEF cells was normalized to the β-actin and arbitrarily set to 1.0. The normalized signal intensities of ppRb and pRb proteins were expressed as a ppRb/pRb ratio. The ratio of ppRb/pRb in passage no. 2 WT MEF cells was arbitrarily set to 1.0. Number denotes passage numbers. *P < 0.05; **P < 0.01.
Figure 8. Analysis of molecular pathways underlying the increased susceptibility of P2Y14-deficient HSPCs to radiation.
(A) WT (red lines) and P2ry14–/– (blue lines) mice were exposed to TBI (6 Gy). Mice (n > 4 mice/genotype) were sacrificed immediately after IR and their LSK (upper) and CD150+CD48– LSK (lower) cells were analyzed for phosphorylated p38 MAPK by flow cytometry. NAC was treated as described. The accompanying graphs (right) show the MFI of phospho–p38 MAPK in LSK (upper) and CD150+CD48– LSK (lower) cells. (B and C) Irradiated WT and KO mice (6 Gy) were sacrificed 8 hours after IR and their LSK and CD150+CD48– LSK cells were analyzed for cell death. Representative dot plots and histograms are shown in Supplemental Figure 10. p38 MAPK inhibitor (SB202190) and JNK inhibitor (SP600125) were administered 30 minutes before and immediately after TBI. (C) Clonogenic capacity of the treated cells was analyzed by CFU assay. (D) P2Y14 KO mice were exposed to TBI (6 Gy). SB202190 and SP600125 were administered as described above. Mice were sacrificed 30 minutes after IR, and their BM cells were transplanted into recipient mice (n > 5/each group). LSK cells were analyzed at 4 to 5 weeks after transplantation for C12FDG expression. P values in Figure 8 were determined using 2-tailed Student’s t test. *P < 0.05; **P < 0.01.
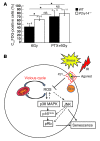
Figure 9. P2Y14 signals through Gi/o to modulate IR-induced HSPC senescense.
(A) Thirty minutes after PTX injection, the mice were subjected to 6 Gy TBI. The BM cells (CD45.2) were immediately harvested and transplanted into recipient mice (CD45.1.2). Recipients were sacrificed 9 to 14 days after transplantation. Donor-derived (CD45.2+) WT and KO LSK cells were analyzed for C12FDG expression. (B) A simplified schema of the proposed model. The P2Y14 receptor couples to Gi/o proteins and inhibits stress-induced (e.g., IR) ROS formation restraining SIPS. In contrast, the high levels of ROS are accumulated in P2Y14-deficient cells by stress and cause mitochondrial dysfunction. This in turn triggers further accumulation of ROS, leading to a vicious cycle. Increased ROS levels mediate the hyperactivation of p38 MAPK, which may in turn mediate the p16/pRb-dependent senescence pathway. JNK activation may potentially be involved in SIPS. Arrows denote activation, and the blunted lines indicate inhibition. Dotted lines denote possible pathways that have not yet been demonstrated. *P < 0.05.
Comment in
- Loss of P2Y₁₄ results in an arresting response to hematological stress.
Garrison BS, Rossi DJ. Garrison BS, et al. J Clin Invest. 2014 Jul;124(7):2846-8. doi: 10.1172/JCI76626. Epub 2014 Jun 17. J Clin Invest. 2014. PMID: 24937422 Free PMC article. - From quiescence to senescence.
Lee BC, Scadden DT. Lee BC, et al. Cell Cycle. 2014;13(22):3469-70. doi: 10.4161/15384101.2014.980696. Cell Cycle. 2014. PMID: 25493411 Free PMC article. No abstract available.
Similar articles
- Loss of P2Y₁₄ results in an arresting response to hematological stress.
Garrison BS, Rossi DJ. Garrison BS, et al. J Clin Invest. 2014 Jul;124(7):2846-8. doi: 10.1172/JCI76626. Epub 2014 Jun 17. J Clin Invest. 2014. PMID: 24937422 Free PMC article. - Empowering human cardiac progenitor cells by P2Y14 nucleotide receptor overexpression.
Khalafalla FG, Kayani W, Kassab A, Ilves K, Monsanto MM, Alvarez R Jr, Chavarria M, Norman B, Dembitsky WP, Sussman MA. Khalafalla FG, et al. J Physiol. 2017 Dec 1;595(23):7135-7148. doi: 10.1113/JP274980. Epub 2017 Nov 9. J Physiol. 2017. PMID: 28980705 Free PMC article. - The expression of P2Y14, a purinergic G-protein coupled receptor, defines functionally distinct subpopulations in placenta-derived hematopoietic stem progenitor cells.
Kook SH, Sim HJ, Lee JC, Lee BC. Kook SH, et al. Leukemia. 2017 Dec;31(12):2837-2841. doi: 10.1038/leu.2017.254. Epub 2017 Aug 14. Leukemia. 2017. PMID: 28804125 No abstract available. - Control of Macrophage Inflammation by P2Y Purinergic Receptors.
Klaver D, Thurnher M. Klaver D, et al. Cells. 2021 May 4;10(5):1098. doi: 10.3390/cells10051098. Cells. 2021. PMID: 34064383 Free PMC article. Review. - Oxidative stress in the regulation of normal and neoplastic hematopoiesis.
Ghaffari S. Ghaffari S. Antioxid Redox Signal. 2008 Nov;10(11):1923-40. doi: 10.1089/ars.2008.2142. Antioxid Redox Signal. 2008. PMID: 18707226 Free PMC article. Review.
Cited by
- Heme oxygenase-1 prevents heart against myocardial infarction by attenuating ischemic injury-induced cardiomyocytes senescence.
Shan H, Li T, Zhang L, Yang R, Li Y, Zhang M, Dong Y, Zhou Y, Xu C, Yang B, Liang H, Gao X, Shan H. Shan H, et al. EBioMedicine. 2019 Jan;39:59-68. doi: 10.1016/j.ebiom.2018.11.056. Epub 2018 Dec 5. EBioMedicine. 2019. PMID: 30527623 Free PMC article. - Bone marrow niche ATP levels determine leukemia-initiating cell activity via P2X7 in leukemic models.
He X, Wan J, Yang X, Zhang X, Huang D, Li X, Zou Y, Chen C, Yu Z, Xie L, Zhang Y, Liu L, Li S, Zhao Y, Shao H, Yu Y, Zheng J. He X, et al. J Clin Invest. 2021 Feb 15;131(4):e140242. doi: 10.1172/JCI140242. J Clin Invest. 2021. PMID: 33301426 Free PMC article. - ADP/P2Y1 aggravates inflammatory bowel disease through ERK5-mediated NLRP3 inflammasome activation.
Zhang C, Qin J, Zhang S, Zhang N, Tan B, Siwko S, Zhang Y, Wang Q, Chen J, Qian M, Liu M, Du B. Zhang C, et al. Mucosal Immunol. 2020 Nov;13(6):931-945. doi: 10.1038/s41385-020-0307-5. Epub 2020 Jun 9. Mucosal Immunol. 2020. PMID: 32518369 - Transcriptomic Analysis of Lung Tissue from Cigarette Smoke-Induced Emphysema Murine Models and Human Chronic Obstructive Pulmonary Disease Show Shared and Distinct Pathways.
Yun JH, Morrow J, Owen CA, Qiu W, Glass K, Lao T, Jiang Z, Perrella MA, Silverman EK, Zhou X, Hersh CP. Yun JH, et al. Am J Respir Cell Mol Biol. 2017 Jul;57(1):47-58. doi: 10.1165/rcmb.2016-0328OC. Am J Respir Cell Mol Biol. 2017. PMID: 28248572 Free PMC article. - Dickkopf-1 promotes hematopoietic regeneration via direct and niche-mediated mechanisms.
Himburg HA, Doan PL, Quarmyne M, Yan X, Sasine J, Zhao L, Hancock GV, Kan J, Pohl KA, Tran E, Chao NJ, Harris JR, Chute JP. Himburg HA, et al. Nat Med. 2017 Jan;23(1):91-99. doi: 10.1038/nm.4251. Epub 2016 Dec 5. Nat Med. 2017. PMID: 27918563 Free PMC article.
References
- Webb TE, Simon J, Bateson AN, Barnard EA. Transient expression of the recombinant chick brain P2y1 purinoceptor and localization of the corresponding mRNA. Cell Mol Biol (Noisy-le-grand). 1994;40(3):437–442. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials