Age-related macular degeneration and changes in the extracellular matrix - PubMed (original) (raw)
Review
Age-related macular degeneration and changes in the extracellular matrix
Małgorzata Nita et al. Med Sci Monit. 2014.
Abstract
Age-related macular degeneration (AMD) is the leading cause of permanent, irreversible, central blindness (scotoma in the central visual field that makes reading and writing impossible, stereoscopic vision, recognition of colors and details) in patients over the age of 50 years in European and North America countries, and an important role is attributed to disorders in the regulation of the extracellular matrix (ECM). The main aim of this article is to present the crucial processes that occur on the level of Bruch's membrane, with special consideration of the metalloproteinase substrates, metalloproteinase, and tissue inhibitor of metalloproteinase (TIMP). A comprehensive review of the literature was performed through MEDLINE and PubMed searches, covering the years 2005-2012, using the following keywords: AMD, extracellular matrix, metalloproteinases, tissue inhibitors of metalloproteinases, Bruch's membrane, collagen, elastin. In the pathogenesis of AMD, a significant role is played by collagen type I and type IV; elastin; fibulin-3, -5, and -6; matrix metalloproteinase (MMP)-2, MMP-9, MMP-14, and MMP-1; and TIMP-3. Other important mechanisms include: ARMS2 and HTR1 proteins, the complement system, the urokinase plasminogen activator system, and pro-renin receptor activation. Continuous rebuilding of the extracellular matrix occurs in both early and advanced AMD, simultaneously with the dysfunction of retinal pigment epithelium (RPE) cells and endothelial cells. The pathological degradation or accumulation of ECM structural components are caused by impairment or hyperactivity of specific MMPs/TIMPs complexes, and is also endangered by the influence of other mechanisms connected with both genetic and environmental factors.
Figures
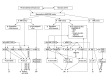
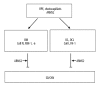
Figure 1
The composition of regular human extracellular matrix (Bruch’s membrane). E/N – entactin/nidogen, PGs – proteoglycans, HSPGs – heparan sulphate proteoglycans.
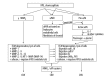
Figure 2
Matrix metalloproteinases (MMP) and tissue inhibitors of metalloproteinases (TIMP) significant in the pathogenesis of early and late AMD. IL-1 – interleukin-1.
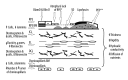
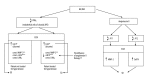
Figure 3
Pathological changes in the retinal pigment epithelium, extracellular matrix, and choroid in age-related macular degeneration. PED - pigment epithelial detachment. * Programmed cell death – apoptosis; ** cell death caused by separation from its BMs – anoikis [14].
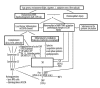
Figure 4
Schematic presentation of the mechanisms causing early and advanced forms of age-related macular degeneration. A2E – bis-retinoid pyrimidine, CEP – carboxyethylpyrrole protein, ILG – isolevuglandin, CFH – complement factor H, FHL – family of factor H-like proteins, DAF – membrane decay-accelerating factor, MCP – membrane cofactor protein, CR – complement receptor, MAC – membrane attack complex, GA – geographical atrophy, PED – pigment epithelium detachment, VEGF – vascular endothelial growth factor, PEDF – pigment epithelium-derived factor, HIF-1 – hypoxia-inducible factor-1.
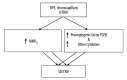
Figure 5
The relation of high-temperature requirement factor A 1 (HTRA 1) to early and advanced age-related macular degeneration. TGFβ – transforming growth factor β.
Figure 6
Protective influence of the age-related maculopathy susceptibility 2 protein (ARMS 2) on the structural and functional elements of the extracellular matrix. FBN – fibulins, FN – fibronectin.
Figure 7
The relationship between the urokinase plasminogen activator receptor/urokinase plasminogen activator system and the development of choroid neovascularization/age-related macular degeneration. IL-1 – interleukin-1, LAMs – laminins, FN1 – fibronectin1.
Figure 8
The influence of arterial hypertension (HA) and activated renin-angiotensin system (RAS) on the development of early age-related macular degeneration. PRRs – pro-renin receptors, LAMs – laminins, AT 1,2 – angiotensin receptors 1,2. * Aggravation effect on AMD by favoring accumulation of coll I, but not by stimulating activity of MMP-2; ** when degrading activity of MMP-2 stays unchanged, the synthesis of coll I increases; *** ↑ activation of PRRs do not stimulate the pro-MMP-2/TIMP-2/MMP-14 complex and therefore coll IV and LAMs, the main causes of SD, remain stable.
References
- Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–73. - PubMed
- Nagase H, Woessner JF. Matrix Metalloproteinases. J Biol Chem. 1999;274(31):21491–94. - PubMed
- Steen B, Sejersen S, Berglin L, et al. Matrix metalloproteinases and metalloproteinase inhibitors in choroidal neovascular membranes. Invest Ophthalmol Vis Sci. 1998;39:2194–200. - PubMed
- Visse R, Nagase H. Matrix Metaloproteinases and tissue inhibitors of metaloproteinases. Circ Res. 2003;92:827–39. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous