Changes in antidepressant use by young people and suicidal behavior after FDA warnings and media coverage: quasi-experimental study - PubMed (original) (raw)
Fang Zhang 2, Matthew D Lakoma 2, Jeanne M Madden 2, Donna Rusinak 2, Robert B Penfold 3, Gregory Simon 4, Brian K Ahmedani 5, Gregory Clarke 6, Enid M Hunkeler 7, Beth Waitzfelder 8, Ashli Owen-Smith 9, Marsha A Raebel 10, Rebecca Rossom 11, Karen J Coleman 12, Laurel A Copeland 13, Stephen B Soumerai 2
Affiliations
- PMID: 24942789
- PMCID: PMC4062705
- DOI: 10.1136/bmj.g3596
Changes in antidepressant use by young people and suicidal behavior after FDA warnings and media coverage: quasi-experimental study
Christine Y Lu et al. BMJ. 2014.
Abstract
Objective: To investigate if the widely publicized warnings in 2003 from the US Food and Drug Administration about a possible increased risk of suicidality with antidepressant use in young people were associated with changes in antidepressant use, suicide attempts, and completed suicides among young people.
Design: Quasi-experimental study assessing changes in outcomes after the warnings, controlling for pre-existing trends.
Setting: Automated healthcare claims data (2000-10) derived from the virtual data warehouse of 11 health plans in the US Mental Health Research Network.
Participants: Study cohorts included adolescents (around 1.1 million), young adults (around 1.4 million), and adults (around 5 million).
Main outcome measures: Rates of antidepressant dispensings, psychotropic drug poisonings (a validated proxy for suicide attempts), and completed suicides.
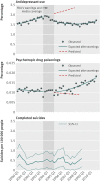
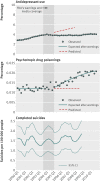
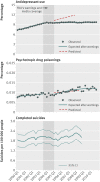
Results: Trends in antidepressant use and poisonings changed abruptly after the warnings. In the second year after the warnings, relative changes in antidepressant use were -31.0% (95% confidence interval -33.0% to -29.0%) among adolescents, -24.3% (-25.4% to -23.2%) among young adults, and -14.5% (-16.0% to -12.9%) among adults. These reflected absolute reductions of 696, 1216, and 1621 dispensings per 100,000 people among adolescents, young adults, and adults, respectively. Simultaneously, there were significant, relative increases in psychotropic drug poisonings in adolescents (21.7%, 95% confidence interval 4.9% to 38.5%) and young adults (33.7%, 26.9% to 40.4%) but not among adults (5.2%, -6.5% to 16.9%). These reflected absolute increases of 2 and 4 poisonings per 100,000 people among adolescents and young adults, respectively (approximately 77 additional poisonings in our cohort of 2.5 million young people). Completed suicides did not change for any age group.
Conclusions: Safety warnings about antidepressants and widespread media coverage decreased antidepressant use, and there were simultaneous increases in suicide attempts among young people. It is essential to monitor and reduce possible unintended consequences of FDA warnings and media reporting.
© Lu et al 2014.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi\_disclosure.pdf (available on request from the corresponding author) and declare that all authors have support from the National Institute of Mental Health for the submitted work. FZ received personal fees from Policy Analysis outside the submitted work. RBP received research funding from Bristol-Meyers Squibb for a study regarding antipsychotic augmentation therapy for major depression in adults only. GS reports grants from Bristol-Myers Squibb outside this work. EH has two pending patents, “Individualized health care management system” (patent application No 11/155 967 publication No US20050283385. Enid Hunkeler, et al. The Permanente Medical Group will own the patent if granted), and “System and method for assisting a care partner in monitoring a patient with chronic disease” (patent application No 11/155 821 publication No US20050283384. Enid Hunkeler, et al. The Permanente Medical Group will own the patent if granted). Other authors declared no financial relationships with any organizations that might have an interest in the submitted work in the previous three years, no other relationships or activities that could appear to have influenced the submitted work.
Figures
Fig 1 Rates of antidepressant use, psychotropic drug poisonings, and completed suicides per quarter before and after the warnings among adolescents enrolled in 11 health plans in nationwide Mental Health Research Network
Fig 2 Rates of antidepressant use, psychotropic drug poisonings, and completed suicides per quarter before and after the warnings among young adults enrolled in 11 health plans in nationwide Mental Health Research Network
Fig 3 Rates of antidepressant use, psychotropic drug poisonings, and completed suicides per quarter before and after the warnings among adults enrolled in 11 health plans in nationwide Mental Health Research Network
Comment in
- Communicating the harmful effects of medicines.
Geddes JR, Cipriani A, Horne R. Geddes JR, et al. BMJ. 2014 Jun 18;348:g4047. doi: 10.1136/bmj.g4047. BMJ. 2014. PMID: 24942829 No abstract available. - Antidepressants increase, rather than decrease, risk of suicidal behaviours in younger patients.
Moore TJ. Moore TJ. BMJ. 2014 Oct 9;349:g5626. doi: 10.1136/bmj.g5626. BMJ. 2014. PMID: 25300283 No abstract available. - Study of study of changes in antidepressant use after FDA warnings is not reliable.
Gøtzsche PC. Gøtzsche PC. BMJ. 2014 Oct 9;349:g5623. doi: 10.1136/bmj.g5623. BMJ. 2014. PMID: 25300434 No abstract available. - Link between FDA antidepressant warnings and increased suicide attempts in young people is questionable.
Olfson M, Schoenbaum M. Olfson M, et al. BMJ. 2014 Oct 9;349:g5614. doi: 10.1136/bmj.g5614. BMJ. 2014. PMID: 25301267 Free PMC article. No abstract available. - Study findings on FDA antidepressant warnings and suicide attempts in young people: a false alarm?
Barber C, Azrael D, Miller M. Barber C, et al. BMJ. 2014 Oct 9;349:g5645. doi: 10.1136/bmj.g5645. BMJ. 2014. PMID: 25301785 No abstract available. - Impossible to draw meaningful conclusions from study of changes in antidepressant use after FDA warnings.
Nardo JM. Nardo JM. BMJ. 2014 Oct 9;349:g5643. doi: 10.1136/bmj.g5643. BMJ. 2014. PMID: 25301791 No abstract available. - Proxy for suicide attempts was inappropriate in study of changes in antidepressant use after FDA warnings.
Bartlett RO. Bartlett RO. BMJ. 2014 Oct 9;349:g5644. doi: 10.1136/bmj.g5644. BMJ. 2014. PMID: 25301794 No abstract available. - More data needed to interpret link between suicide and FDA warning on antidepressants.
Case BG. Case BG. BMJ. 2014 Oct 9;349:g5616. doi: 10.1136/bmj.g5616. BMJ. 2014. PMID: 25301797 No abstract available. - Authors' reply to Olfson and Schoenbaum, Nardo, Bartlett, Moore, Case, Gøtzsche, and Barber and colleagues.
Lu CY, Simon G, Soumerai SB. Lu CY, et al. BMJ. 2014 Oct 9;349:g5722. doi: 10.1136/bmj.g5722. BMJ. 2014. PMID: 25301799 No abstract available. - Authors' reply to Mosholder and colleagues.
Lu CY, Simon G, Soumerai SB. Lu CY, et al. BMJ. 2014 Oct 29;349:g6516. doi: 10.1136/bmj.g6516. BMJ. 2014. PMID: 25354502 No abstract available. - Reservations about study on antidepressant use by young people and suicidal behaviour after FDA warnings.
Mosholder AD, Taylor LG, Crentsil V. Mosholder AD, et al. BMJ. 2014 Oct 29;349:g6503. doi: 10.1136/bmj.g6503. BMJ. 2014. PMID: 25355684 No abstract available.
Similar articles
- Intended And Unintended Outcomes After FDA Pediatric Antidepressant Warnings: A Systematic Review.
Soumerai SB, Koppel R, Naci H, Madden JM, Fry A, Halbisen A, Angeles J, Koppel J, Rubin R, Lu CY. Soumerai SB, et al. Health Aff (Millwood). 2024 Oct;43(10):1360-1369. doi: 10.1377/hlthaff.2023.00263. Health Aff (Millwood). 2024. PMID: 39374452 - Link between FDA antidepressant warnings and increased suicide attempts in young people is questionable.
Olfson M, Schoenbaum M. Olfson M, et al. BMJ. 2014 Oct 9;349:g5614. doi: 10.1136/bmj.g5614. BMJ. 2014. PMID: 25301267 Free PMC article. No abstract available. - Study findings on FDA antidepressant warnings and suicide attempts in young people: a false alarm?
Barber C, Azrael D, Miller M. Barber C, et al. BMJ. 2014 Oct 9;349:g5645. doi: 10.1136/bmj.g5645. BMJ. 2014. PMID: 25301785 No abstract available. - Reservations about study on antidepressant use by young people and suicidal behaviour after FDA warnings.
Mosholder AD, Taylor LG, Crentsil V. Mosholder AD, et al. BMJ. 2014 Oct 29;349:g6503. doi: 10.1136/bmj.g6503. BMJ. 2014. PMID: 25355684 No abstract available. - Antidepressant Prescribing and Suicide/Self-Harm by Young Australians: Regulatory Warnings, Contradictory Advice, and Long-Term Trends.
Whitely M, Raven M, Jureidini J. Whitely M, et al. Front Psychiatry. 2020 Jun 5;11:478. doi: 10.3389/fpsyt.2020.00478. eCollection 2020. Front Psychiatry. 2020. PMID: 32587531 Free PMC article. Review.
Cited by
- The Impact of the Massachusetts Behavioral Health Child Screening Policy on Service Utilization.
Hacker K, Penfold R, Arsenault LN, Zhang F, Soumerai SB, Wissow LS. Hacker K, et al. Psychiatr Serv. 2017 Jan 1;68(1):25-32. doi: 10.1176/appi.ps.201500543. Epub 2016 Sep 1. Psychiatr Serv. 2017. PMID: 27582240 Free PMC article. - Psychotropic Medication Use among Insured Children with Autism Spectrum Disorder.
Madden JM, Lakoma MD, Lynch FL, Rusinak D, Owen-Smith AA, Coleman KJ, Quinn VP, Yau VM, Qian YX, Croen LA. Madden JM, et al. J Autism Dev Disord. 2017 Jan;47(1):144-154. doi: 10.1007/s10803-016-2946-7. J Autism Dev Disord. 2017. PMID: 27817163 Free PMC article. - Ethical and Practical Considerations in Removing Black Box Warnings from Drug Labels.
Yeh JS, Sarpatwari A, Kesselheim AS. Yeh JS, et al. Drug Saf. 2016 Aug;39(8):709-14. doi: 10.1007/s40264-016-0419-8. Drug Saf. 2016. PMID: 27000800 - Recent Advances in Means Safety as a Suicide Prevention Strategy.
Jin HM, Khazem LR, Anestis MD. Jin HM, et al. Curr Psychiatry Rep. 2016 Oct;18(10):96. doi: 10.1007/s11920-016-0731-0. Curr Psychiatry Rep. 2016. PMID: 27629355 Review. - Measuring the impact of pharmacovigilance activities, challenging but important.
van Hunsel F, Gardarsdottir H, de Boer A, Kant A. van Hunsel F, et al. Br J Clin Pharmacol. 2019 Oct;85(10):2235-2237. doi: 10.1111/bcp.14042. Epub 2019 Jul 31. Br J Clin Pharmacol. 2019. PMID: 31368147 Free PMC article. No abstract available.
References
- Kaizar EE, Greenhouse JB, Seltman H, Kelleher K. Do antidepressants cause suicidality in children? A Bayesian meta-analysis. Clin Trials 2006;3:73-90; discussion 91-78. - PubMed
- Leckman JF, King RA. A developmental perspective on the controversy surrounding the use of SSRIs to treat pediatric depression. Am J Psychiatry 2007;164:1304-6. - PubMed
- Simon GE. The antidepressant quandary—considering suicide risk when treating adolescent depression. N Engl J Med 2006;355:2722-3. - PubMed
- Hammad TA, Laughren T, Racoosin J. Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry 2006;63:332-9. - PubMed
- Olfson M, Marcus SC. A case-control study of antidepressants and attempted suicide during early phase treatment of major depressive episodes. J Clin Psychiatry 2008;69:425-32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 DK092924/DK/NIDDK NIH HHS/United States
- U19 MH092201/MH/NIMH NIH HHS/United States
- U19MH092201/MH/NIMH NIH HHS/United States
- 1P30-DK092924/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical