TRF2, but not TBP, mediates the transcription of ribosomal protein genes - PubMed (original) (raw)
TRF2, but not TBP, mediates the transcription of ribosomal protein genes
Yuan-Liang Wang et al. Genes Dev. 2014.
Abstract
The TCT core promoter element is present in most ribosomal protein (RP) genes in Drosophila and humans. Here we show that TBP (TATA box-binding protein)-related factor TRF2, but not TBP, is required for transcription of the TCT-dependent RP genes. In cells, TCT-dependent transcription, but not TATA-dependent transcription, increases or decreases upon overexpression or depletion of TRF2. In vitro, purified TRF2 activates TCT but not TATA promoters. ChIP-seq (chromatin immunoprecipitation [ChIP] combined with deep sequencing) experiments revealed the preferential localization of TRF2 at TCT versus TATA promoters. Hence, a specialized TRF2-based RNA polymerase II system functions in the synthesis of RPs and complements the RNA polymerase I and III systems.
Keywords: RNA polymerase II; TCT motif; TRF2; core promoter; ribosomal protein genes.
© 2014 Wang et al.; Published by Cold Spring Harbor Laboratory Press.
Figures
Figure 1.
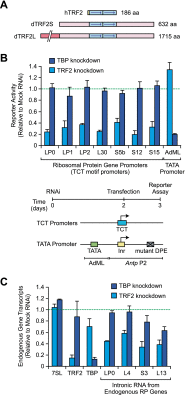
TCT-dependent transcription appears to require TRF2 but not TBP. (A) Schematic diagrams of hTRF2 and the two forms of Drosophila TRF2 (dTRF2S and dTRF2L). dTRF2S is identical to the C-terminal 632-amino-acid residues of dTRF2L. (B) Depletion of TRF2, but not TBP, reduces RP gene expression. Drosophila S2 cells were depleted of either TRF2 or TBP by RNAi and then transfected with TCT-dependent or TATA-dependent luciferase reporter genes. The experimental scheme and reporter constructs are depicted at the bottom of the figure. The activities of the RNAi-depleted extracts are reported as relative to the activities of mock RNAi-treated control extracts. Error bars represent the standard deviation. (C) Analysis of endogenous transcript levels by qRT–PCR. Drosophila S2 cells were depleted of TRF2 or TBP, as in B. The total RNA was then isolated and analyzed by qRT–PCR. For the RP genes, intronic sequences were used to detect newly synthesized transcripts. We did not analyze RP genes with intronic snoRNA genes, as they could affect the levels of the intronic RNAs. The error bars represent the standard deviation.
Figure 2.
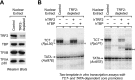
Purified TRF2 is required for in vitro transcription of TCT-dependent genes but not a TATA-dependent gene. (A) Immunodepletion of endogenous dTRF2 from a Drosophila embryo nuclear extract. The levels of TRF2 (dTRF2S), TBP, TFIIB, and TFIIA (p30 subunit) in TRF2-depleted extracts versus control extracts were monitored by Western blot analysis. We were able to detect dTRF2S but not dTRF2L even though the antibodies were raised against a polypeptide that is shared by both proteins. This effect may be due to inefficient transfer of the dTRF2L protein to the blot, the lack of recognition of dTRF2L by the antibodies, or the absence of dTRF2L in the extract used in the Western blot. (B) Purified hTRF2, but not purified hTBP, is able to restore the specific loss of TCT-dependent transcription that occurs upon depletion of TRF2 from a nuclear extract. Two-template in vitro transcription assays were performed with TCT-dependent and TATA-dependent promoters. Reactions were carried out with either TRF2-depleted or control nuclear extracts. Where indicated, purified hTRF2 or hTBP was added to reactions with the TRF2-depleted extracts. The resulting transcripts were detected by primer extension–reverse transcription analysis. The single asterisk denotes a nonspecific transcript. The double asterisk indicates a nonspecific transcript that is observed in the presence of hTBP. This stimulation of nonspecific initiation by hTBP is most likely a consequence of the relatively nonspecific binding of hTBP to DNA. This effect was not observed with dTBP (Supplemental Fig. S7).
Figure 3.
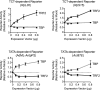
Overexpression of TRF2 increases TCT-dependent but not TATA-dependent transcription, whereas overexpression of TBP increases TATA-dependent but not TCT-dependent transcription. Drosophila S2 cells were transfected with a TCT-dependent or TATA-dependent reporter construct along with the indicated amounts of expression vector for either dTRF2S or dTBP. The AdML-_Antp_P2 promoter is identical to the TATA promoter that was used in Figure 1B. Luciferase reporter activities were normalized to those obtained with the empty vector alone. Error bars represent the standard deviation.
Figure 4.
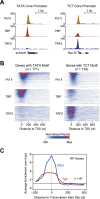
TRF2 is enriched at TCT-dependent promoters in vivo. The occupancy of TRF2 and TBP was analyzed by ChIP-seq experiments with Drosophila embryos collected from 2 to 4 h after egg deposition. (A) Differential TRF2 occupancy at a TATA promoter and a TCT promoter. Read counts across a representative TATA-containing promoter (achaete) and a representative TCT-containing promoter (RpL30) with comparable levels of RNA Pol II show that TRF2 is bound at higher levels to the TCT promoter relative to the TATA promoter. (B) Heat maps of the ChIP occupancy of Pol II, TRF2, and TBP at 171 genes with a predicted TATA box and at 134 genes with a predicted TCT motif. These two sets of genes were sorted in descending order of Pol II occupancy and are shown in a window from −200 to + 800 nt relative to the +1 transcription start site. The same genes and their order are shown for Pol II, TBP, and TRF2 occupancy. (C) TRF2 occupancy is highest near the transcription start site of RP genes. The graph depicts the average enrichments of the TRF2 and TBP ChIP-seq signals over input from −250 to +500 nt relative to the transcription start site for the 87 known RP genes. The dashed line indicates the peak TRF2 signal at −3 nt relative to the transcription start site.
Similar articles
- TRF2: TRansForming the view of general transcription factors.
Zehavi Y, Kedmi A, Ideses D, Juven-Gershon T. Zehavi Y, et al. Transcription. 2015;6(1):1-6. doi: 10.1080/21541264.2015.1004980. Epub 2015 Jan 14. Transcription. 2015. PMID: 25588059 Free PMC article. Review. - Drosophila TRF2 is a preferential core promoter regulator.
Kedmi A, Zehavi Y, Glick Y, Orenstein Y, Ideses D, Wachtel C, Doniger T, Waldman Ben-Asher H, Muster N, Thompson J, Anderson S, Avrahami D, Yates JR 3rd, Shamir R, Gerber D, Juven-Gershon T. Kedmi A, et al. Genes Dev. 2014 Oct 1;28(19):2163-74. doi: 10.1101/gad.245670.114. Epub 2014 Sep 15. Genes Dev. 2014. PMID: 25223897 Free PMC article. - Role of the TATA-box binding protein (TBP) and associated family members in transcription regulation.
Mishal R, Luna-Arias JP. Mishal R, et al. Gene. 2022 Jul 30;833:146581. doi: 10.1016/j.gene.2022.146581. Epub 2022 May 18. Gene. 2022. PMID: 35597524 Review. - TRF2 associates with DREF and directs promoter-selective gene expression in Drosophila.
Hochheimer A, Zhou S, Zheng S, Holmes MC, Tjian R. Hochheimer A, et al. Nature. 2002 Nov 28;420(6914):439-45. doi: 10.1038/nature01167. Nature. 2002. PMID: 12459787 - A sequence-specific core promoter-binding transcription factor recruits TRF2 to coordinately transcribe ribosomal protein genes.
Baumann DG, Gilmour DS. Baumann DG, et al. Nucleic Acids Res. 2017 Oct 13;45(18):10481-10491. doi: 10.1093/nar/gkx676. Nucleic Acids Res. 2017. PMID: 28977400 Free PMC article.
Cited by
- A unified view of the sequence and functional organization of the human RNA polymerase II promoter.
Luse DS, Parida M, Spector BM, Nilson KA, Price DH. Luse DS, et al. Nucleic Acids Res. 2020 Aug 20;48(14):7767-7785. doi: 10.1093/nar/gkaa531. Nucleic Acids Res. 2020. PMID: 32597978 Free PMC article. - Regulation of ribosomal protein genes: An ordered anarchy.
Petibon C, Malik Ghulam M, Catala M, Abou Elela S. Petibon C, et al. Wiley Interdiscip Rev RNA. 2021 May;12(3):e1632. doi: 10.1002/wrna.1632. Epub 2020 Oct 10. Wiley Interdiscip Rev RNA. 2021. PMID: 33038057 Free PMC article. Review. - Comparative cofactor screens show the influence of transactivation domains and core promoters on the mechanisms of transcription.
Bell CC, Balic JJ, Talarmain L, Gillespie A, Scolamiero L, Lam EYN, Ang CS, Faulkner GJ, Gilan O, Dawson MA. Bell CC, et al. Nat Genet. 2024 Jun;56(6):1181-1192. doi: 10.1038/s41588-024-01749-z. Epub 2024 May 20. Nat Genet. 2024. PMID: 38769457 - The core-promoter factor TRF2 mediates a Fruitless action to masculinize neurobehavioral traits in Drosophila.
Chowdhury ZS, Sato K, Yamamoto D. Chowdhury ZS, et al. Nat Commun. 2017 Nov 14;8(1):1480. doi: 10.1038/s41467-017-01623-z. Nat Commun. 2017. PMID: 29133872 Free PMC article. - Evolution and diversification of the basal transcription machinery.
Duttke SH. Duttke SH. Trends Biochem Sci. 2015 Mar;40(3):127-9. doi: 10.1016/j.tibs.2015.01.005. Epub 2015 Feb 5. Trends Biochem Sci. 2015. PMID: 25661246 Free PMC article. Review.
References
- Cormack BP, Struhl K 1992. The TATA-binding protein is required for transcription by all three nuclear RNA polymerases in yeast cells. Cell 69: 685–696 - PubMed
- Dantonel JC, Quintin S, Lakatos L, Labouesse M, Tora L 2000. TBP-like factor is required for embryonic RNA polymerase II transcription in C. elegans. Mol Cell 6: 715–722 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous