Systemic inflammation impairs attention and cognitive flexibility but not associative learning in aged rats: possible implications for delirium - PubMed (original) (raw)
Systemic inflammation impairs attention and cognitive flexibility but not associative learning in aged rats: possible implications for delirium
Deborah J Culley et al. Front Aging Neurosci. 2014.
Abstract
Delirium is a common and morbid condition in elderly hospitalized patients. Its pathophysiology is poorly understood but inflammation has been implicated based on a clinical association with systemic infection and surgery and preclinical data showing that systemic inflammation adversely affects hippocampus-dependent memory. However, clinical manifestations and imaging studies point to abnormalities not in the hippocampus but in cortical circuits. We therefore tested the hypothesis that systemic inflammation impairs prefrontal cortex function by assessing attention and executive function in aged animals. Aged (24-month-old) Fischer-344 rats received a single intraperitoneal injection of lipopolysaccharide (LPS; 50 μg/kg) or saline and were tested on the attentional set-shifting task (AST), an index of integrity of the prefrontal cortex, on days 1-3 post-injection. Plasma and frontal cortex concentrations of the cytokine TNFα and the chemokine CCL2 were measured by ELISA in separate groups of identically treated, age-matched rats. LPS selectively impaired reversal learning and attentional shifts without affecting discrimination learning in the AST, indicating a deficit in attention and cognitive flexibility but not learning globally. LPS increased plasma TNFα and CCL2 acutely but this resolved within 24-48 h. TNFα in the frontal cortex did not change whereas CCL2 increased nearly threefold 2 h after LPS but normalized by the time behavioral testing started 24 h later. Together, our data indicate that systemic inflammation selectively impairs attention and executive function in aged rodents and that the cognitive deficit is independent of concurrent changes in frontal cortical TNFα and CCL2. Because inattention is a prominent feature of clinical delirium, our data support a role for inflammation in the pathogenesis of this clinical syndrome and suggest this animal model could be useful for studying that relationship further.
Keywords: CCL2; aging neuroscience; frontal cortex; inflammation; lipopolysaccharides; rats; set-shifting.
Figures
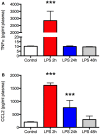
Figure 1
Lipopolysaccharide (LPS) produces a robust but transient increase in plasma TNFα and CCL2. Plasma was sampled at the time of sacrifice from aged Fisher 344 rats 2, 24, and 48 h after administration of 50 μg/kg LPS (N = 5 per group) or a control group (N = 5) that received an equal volume of saline intraperitoneally. There was a marked increase in both TNFα (A) and CCL2 (B) 2 h after LPS but the effect resolved completely by 24 or 48 h, respectively. Data are mean ± SEM. ***P < 0.001 by one-way ANOVA.
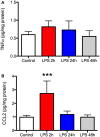
Figure 2
Lipopolysaccharide (LPS) produces a robust but transient increase in TNFα and CCL2 in the frontal cortex. Rats (N = 5 per group) were treated as described in Figure 1 and frontal cortex harvested at the time of sacrifice. There was no change in TNFα in the frontal cortex at any time compared to control animals (A). CCL2 was elevated nearly threefold above control 2 h after LPS but this resolved by 24 h after treatment (B). Data are mean ± SEM. ***P < 0.001 by one-way ANOVA.
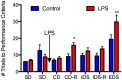
Figure 3
Lipopolysaccharide selectively impaired attention/executive function in aged rats. Twenty-four-month-old Fischer-344 rats (N = 11) were tested on two simple discrimination (SD) tasks, one from each dimension (medium and shape) prior to receiving LPS 50 μg/kg or an equal volume of saline i.p. They were tested on the compound discrimination (CD) task and compound discrimination reversal (CD-R) on day 1 after LPS, the intradimensional shift (IDS) and intradimensional shift reversal (IDS-R) on day 2 after LPS, and the extradimensional shift (EDS) on day 3 after LPS. There were no differences between the groups at baseline. LPS did not affect performance on the CD or IDS task but impaired performance on the CD-R and the EDS. This indicates LPS had no effect on simple discrimination learning but did impair attention/executive function for at least 3 days. Data are mean ± SEM. *P ≤ 0.05, **P ≤ 0.01 by two-way ANOVA.
Similar articles
- In a Model of Neuroinflammation Designed to Mimic Delirium, Quetiapine Reduces Cortisol Secretion and Preserves Reversal Learning in the Attentional Set Shifting Task.
Carr ZJ, Miller L, Ruiz-Velasco V, Kunselman AR, Karamchandani K. Carr ZJ, et al. J Neuroimmune Pharmacol. 2019 Sep;14(3):383-390. doi: 10.1007/s11481-019-09857-y. Epub 2019 May 22. J Neuroimmune Pharmacol. 2019. PMID: 31119596 - Effect of Systemic Inflammation on Rat Attentional Function and Neuroinflammation: Possible Protective Role for Food Restriction.
Yegla B, Foster T. Yegla B, et al. Front Aging Neurosci. 2019 Oct 25;11:296. doi: 10.3389/fnagi.2019.00296. eCollection 2019. Front Aging Neurosci. 2019. PMID: 31708767 Free PMC article. - Aged rats are impaired on an attentional set-shifting task sensitive to medial frontal cortex damage in young rats.
Barense MD, Fox MT, Baxter MG. Barense MD, et al. Learn Mem. 2002 Jul-Aug;9(4):191-201. doi: 10.1101/lm.48602. Learn Mem. 2002. PMID: 12177232 Free PMC article. - Effects of stress on behavioral flexibility in rodents.
Hurtubise JL, Howland JG. Hurtubise JL, et al. Neuroscience. 2017 Mar 14;345:176-192. doi: 10.1016/j.neuroscience.2016.04.007. Epub 2016 Apr 9. Neuroscience. 2017. PMID: 27066767 Review. - [Prefrontal cortex in memory and attention processes].
Allegri RF, Harris P. Allegri RF, et al. Rev Neurol. 2001 Mar 1-15;32(5):449-53. Rev Neurol. 2001. PMID: 11426408 Review. Spanish.
Cited by
- The potential mechanism of mitochondrial homeostasis in postoperative neurocognitive disorders: an in-depth review.
Ye F, Wei C, Wu A. Ye F, et al. Ann Med. 2024 Dec;56(1):2411012. doi: 10.1080/07853890.2024.2411012. Epub 2024 Oct 25. Ann Med. 2024. PMID: 39450938 Free PMC article. Review. - Accelerated aging in people living with HIV: The neuroimmune feedback model.
Schrock JM. Schrock JM. Brain Behav Immun Health. 2024 Feb 5;36:100737. doi: 10.1016/j.bbih.2024.100737. eCollection 2024 Mar. Brain Behav Immun Health. 2024. PMID: 38356933 Free PMC article. - Exploring the Pathophysiology of Delirium: An Overview of Biomarker Studies, Animal Models, and Tissue-Engineered Models.
McKay TB, Khawaja ZQ, Freedman IG, Turco I, Wiredu K, Colecchi T, Akeju O. McKay TB, et al. Anesth Analg. 2023 Dec 1;137(6):1186-1197. doi: 10.1213/ANE.0000000000006715. Epub 2023 Oct 18. Anesth Analg. 2023. PMID: 37851904 Review. - Preclinical and translational models for delirium: Recommendations for future research from the NIDUS delirium network.
Vasunilashorn SM, Lunardi N, Newman JC, Crosby G, Acker L, Abel T, Bhatnagar S, Cunningham C, de Cabo R, Dugan L, Hippensteel JA, Ishizawa Y, Lahiri S, Marcantonio ER, Xie Z, Inouye SK, Terrando N, Eckenhoff RG; NIDUS Delirium Network. Vasunilashorn SM, et al. Alzheimers Dement. 2023 May;19(5):2150-2174. doi: 10.1002/alz.12941. Epub 2023 Feb 17. Alzheimers Dement. 2023. PMID: 36799408 Free PMC article. Review. - Immune cell counts in cerebrospinal fluid predict cognitive function in aging and neurodegenerative disease.
Snyder A, Grant H, Chou A, Lindbergh CA, Kramer JH, Miller BL, Elahi FM. Snyder A, et al. Alzheimers Dement. 2023 Aug;19(8):3339-3349. doi: 10.1002/alz.12956. Epub 2023 Feb 15. Alzheimers Dement. 2023. PMID: 36791265 Free PMC article.
References
- Brooks J. M., Pershing M. L., Thomsen M. S., Mikkelsen J. D., Sarter M., Bruno J.P. (2012). Transient inactivation of the neonatal ventral hippocampus impairs attentional set-shifting behavior: reversal with an α7 nicotinic agonist. Neuropsychopharmacology 37, 2476–248610.1038/npp.2012.106 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources