Tissue- and cell-specific mitochondrial defect in Parkin-deficient mice - PubMed (original) (raw)
. 2014 Jun 24;9(6):e99898.
doi: 10.1371/journal.pone.0099898. eCollection 2014.
Clément A Gautier 1, Anne-Laure Bulteau 2, Rosa Ferrando-Miguel 1, Caroline Gouarne 3, Marc Giraudon Paoli 3, Rebecca Pruss 3, Françoise Auchère 4, Caroline L'Hermitte-Stead 2, Frédéric Bouillaud 2, Alexis Brice 5, Olga Corti 1, Anne Lombès 2
Affiliations
- PMID: 24959870
- PMCID: PMC4069072
- DOI: 10.1371/journal.pone.0099898
Tissue- and cell-specific mitochondrial defect in Parkin-deficient mice
Maria Damiano et al. PLoS One. 2014.
Abstract
Loss of Parkin, encoded by PARK2 gene, is a major cause of autosomal recessive Parkinson's disease. In Drosophila and mammalian cell models Parkin has been shown in to play a role in various processes essential to maintenance of mitochondrial quality, including mitochondrial dynamics, biogenesis and degradation. However, the relevance of altered mitochondrial quality control mechanisms to neuronal survival in vivo is still under debate. We addressed this issue in the brain of PARK2-/- mice using an integrated mitochondrial evaluation, including analysis of respiration by polarography or by fluorescence, respiratory complexes activity by spectrophotometric assays, mitochondrial membrane potential by rhodamine 123 fluorescence, mitochondrial DNA content by real time PCR, and oxidative stress by total glutathione measurement, proteasome activity, SOD2 expression and proteins oxidative damage. Respiration rates were lowered in PARK2-/- brain with high resolution but not standard respirometry. This defect was specific to the striatum, where it was prominent in neurons but less severe in astrocytes. It was present in primary embryonic cells and did not worsen in vivo from 9 to 24 months of age. It was not associated with any respiratory complex defect, including complex I. Mitochondrial inner membrane potential in PARK2-/- mice was similar to that of wild-type mice but showed increased sensitivity to uncoupling with ageing in striatum. The presence of oxidative stress was suggested in the striatum by increased mitochondrial glutathione content and oxidative adducts but normal proteasome activity showed efficient compensation. SOD2 expression was increased only in the striatum of PARK2-/- mice at 24 months of age. Altogether our results show a tissue-specific mitochondrial defect, present early in life of PARK2-/- mice, mildly affecting respiration, without prominent impact on mitochondrial membrane potential, whose underlying mechanisms remain to be elucidated, as complex I defect and prominent oxidative damage were ruled out.
Conflict of interest statement
Competing Interests: CG & RP are employees of Trophos. There are no patents, products in development or marketed products to declare. This does not alter the authors' adherence to all the PLOS ONE policies on sharing data and materials.
Figures
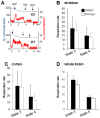
Figure 1. Respiration was similar in brain mitochondrial preparations from 24-month-old PARK2−/− and wild type mice examined with standard oxygraphy.
(A) Representative experiment with a crude mitochondrial pellet from striatum. Blue curve = oxygen (O2) concentration expressed as nmol/mL; red curve = rate of oxygen consumption expressed as nmoles O2/mL and min; ADP = addition of 400 µM ADP; OM = addition of 1 µg/mL oligomycin, a complex V inhibitor; KCN = addition of 1 mM KCN (non-respiratory oxygen consumption); (B, C, D) Bars showing state 3 respiration (respiration in the presence of substrates +ADP – non-respiratory oxygen consumption) and state 4 respiration (respiration in the presence of oligomycin – non-respiratory oxygen consumption) on complex I substrates (glutamate+malate); black bars = PARK2−/−, white bars = wild type; data are expressed as nmoles O2/mL and mg proteins and are shown as means ± SD; two animals, one per genotype, were analyzed the same day; the numbers between brackets indicate the number of individual animals of each genotype; (B) results from crude mitochondrial pellets from striatum (n = 6), (C) crude mitochondrial pellets from cortex (n = 7) and (D) purified mitochondria from whole brain (n = 2).
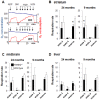
Figure 2. High resolution respirometry reveals a respiration defect in PARK2−/− mice.
(A) Representative experiment with striatal post-nuclear supernatant. Blue curve = oxygen concentration (O2) expressed as nmol/mL; red curve = rate of oxygen consumption expressed as nmoles O2/mL and min; successive additions: ADP = 1 mM ADP in the medium with 10 mM glutamate+5 mM malate; OM = 1 µg/mL oligomycin, cccp = successive additions of 1.25 µM (up to the maximal respiration rate independent from ATP synthase capacity); KCN = 1 mM KCN (non-respiratory oxygen consumption); (B, C, D) Bars showing state 3 respiration, state 4 respiration and respiratory reserve on complex I substrates (glutamate+malate) in post-nuclear supernatants from (B) striatum, (C) midbrain and (D) liver of 24- and 9-month-old PARK2−/− (black bars) and wild type mice (white bars); all data are expressed as nmoles O2/mL and mg proteins and shown as means ± SD; two animals, one per genotype, were analyzed the same day; the data were obtained from 6 to 8 individual animals of each genotype. * p<0.05 using Mann and Whitney test. In liver, state 3 respiration rate and respiratory reserve significantly decreased with age in both wild type mice (p = 0.011 and <0.001 when comparing state 3 respiration and respiratory reserve respectively between 9 and 24 months of age using Mann and Whitney test) and in PARK2−/− mice (p = 0.019 and <0.001 when comparing state 3 respiration and respiratory reserve respectively between 9 and 24 months of age using Mann and Whitney test).
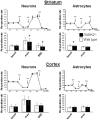
Figure 3. Maximal respiration is reduced in striatal neurons from PARK2−/− mice.
Representative experiments with neurons or astrocytes from striatum or cortex: the traces represent the evolution of the respiration rate (in pmol O2/minute) in Seahorse plates seeded with cells from PARK2−/− (black circles) and wild type (white circles) mice. Sequential additions are: oligomycin (OM) (0.25 µg/ml for neurons and 0.5 µg/ml for astrocytes), fccp (FP) (3 µM for cortical neurons and 1 µM for other cells), and rotenone+antimycine (RA) (50 nM rotenone+150 µg/ml antimycin A). Bars below the traces show the means and SD of basal respiration, maximal respiration and spare respiratory capacity (SRC = maximal – basal respiration) of 3 independent tests, each performed in 6 to 12 independent wells. * p<0.05 using Mann and Whitney test.
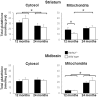
Figure 4. The inner mitochondrial membrane potential is normal in midbrain and striatum of PARK2 −/− mice.
Dark circles = PARK2−/−, white circles = wild type; ΔΨm = inner mitochondrial membrane potential expressed as mV after transformation of the fluorescent rhodamine 123 signal using the Nernst equation, as explained in the methods section; A = ΔΨm in the presence of glutamate+malate; B = A+1 mM ADP; C = B+1 µM oligomycin; D = C+1 µM cccp; E = C+2 µM cccp; F = C+4 µM cccp;G = C+8 µM cccp. The data were obtained in parallel to the analysis of respiration; two animals, one per genotype, were analyzed the same day; the data obtained from seven 9-month-old mice and six 24-month-old ones of each genotype, they are expressed as mean and SD.
Figure 5. Increase of the mitochondrial glutathione content in striatum of 12-month-old PARK2 −/− mice.
Total glutathione (GSH+GS-SG) levels were determined in cytosols and isolated mitochondria from ventral midbrain and striatum of 12- and 24-month-old PARK2−/− (black bars) and wild-type mice (white bars), data are expressed as mean and SD of five independent measurements for each age and each genotype. * = p<0.05 using multiple comparison with Holm-Sidak test. Significant differences with age, without influence of the genotype, were observed for midbrain mitochondrial and striatal cytosolic glutathione content. Significant interaction between age and genotype was present for striatal mitochondrial glutathione content, which significantly increased with age only in wild type mice and was at 12 months of age significantly higher in PARK2−/− than in wild type mice.
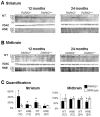
Figure 6. Presence of oxidative adducts in striatal crude mitochondrial pellets from PARK2 −/− mice.
Western blot analysis of proteins from crude mitochondrial pellets from striatum (A) and midbrain (B) of PARK2 −/− and wild-type mice, at 12 and 24 months of age; loading control was the outer membrane protein Voltage-Dependent Anion Channel (VDAC); (C) densitometric analysis of oxidative adducts was performed in the length of the membrane shown in A and B, that signal was then normalized to VDAC signal and quantification was expressed as means and SD and as % of the mean of wild-type samples on the membrane shown in A and B; * = p<0.05 using Mann and Whitney test.
Figure 7. Increased mitochondrial Mn superoxide dismutase (SOD2) in the striatum of PARK2 −/− mice.
Western blot analysis of proteins from crude mitochondrial pellets from striatum (A) and midbrain (B) of PARK2 −/− and wild-type mice, at 12 and 24 months of age; loading control was the outer membrane protein Voltage-Dependent Anion Channel (VDAC); (C) densitometric analysis of the SOD2 signal shown in A and B was normalized to VDAC signal and quantification was expressed as means and SD and as % of the mean of wild-type samples on the membrane shown in A and B; * = p<0.05 using Mann and Whitney test; † and ‡: samples from 12-month-old PARK2−/− and wild type mice respectively showing that the SOD2 steady-state level does not significantly change with age.
Similar articles
- Protective role of Parkin in skeletal muscle contractile and mitochondrial function.
Gouspillou G, Godin R, Piquereau J, Picard M, Mofarrahi M, Mathew J, Purves-Smith FM, Sgarioto N, Hepple RT, Burelle Y, Hussain SNA. Gouspillou G, et al. J Physiol. 2018 Jul;596(13):2565-2579. doi: 10.1113/JP275604. Epub 2018 May 30. J Physiol. 2018. PMID: 29682760 Free PMC article. - [Etiology and pathogenesis of Parkinson's disease: from mitochondrial dysfunctions to familial Parkinson's disease].
Hattori N. Hattori N. Rinsho Shinkeigaku. 2004 Apr-May;44(4-5):241-62. Rinsho Shinkeigaku. 2004. PMID: 15287506 Review. Japanese. - Behavioral and neurotransmitter abnormalities in mice deficient for Parkin, DJ-1 and superoxide dismutase.
Hennis MR, Seamans KW, Marvin MA, Casey BH, Goldberg MS. Hennis MR, et al. PLoS One. 2013 Dec 26;8(12):e84894. doi: 10.1371/journal.pone.0084894. eCollection 2013. PLoS One. 2013. PMID: 24386432 Free PMC article. - Glutamate excitotoxicity in neurons triggers mitochondrial and endoplasmic reticulum accumulation of Parkin, and, in the presence of N-acetyl cysteine, mitophagy.
Van Laar VS, Roy N, Liu A, Rajprohat S, Arnold B, Dukes AA, Holbein CD, Berman SB. Van Laar VS, et al. Neurobiol Dis. 2015 Feb;74:180-93. doi: 10.1016/j.nbd.2014.11.015. Epub 2014 Dec 3. Neurobiol Dis. 2015. PMID: 25478815 Free PMC article. - Impaired mitochondrial dynamics and function in the pathogenesis of Parkinson's disease.
Büeler H. Büeler H. Exp Neurol. 2009 Aug;218(2):235-46. doi: 10.1016/j.expneurol.2009.03.006. Epub 2009 Mar 18. Exp Neurol. 2009. PMID: 19303005 Review.
Cited by
- Mitochondrial DNA depletion by ethidium bromide decreases neuronal mitochondrial creatine kinase: Implications for striatal energy metabolism.
Warren EB, Aicher AE, Fessel JP, Konradi C. Warren EB, et al. PLoS One. 2017 Dec 29;12(12):e0190456. doi: 10.1371/journal.pone.0190456. eCollection 2017. PLoS One. 2017. PMID: 29287112 Free PMC article. - Presenilin-Deficient Neurons and Astrocytes Display Normal Mitochondrial Phenotypes.
Contino S, Suelves N, Vrancx C, Vadukul DM, Payen VL, Stanga S, Bertrand L, Kienlen-Campard P. Contino S, et al. Front Neurosci. 2021 Jan 22;14:586108. doi: 10.3389/fnins.2020.586108. eCollection 2020. Front Neurosci. 2021. PMID: 33551720 Free PMC article. - Mitochondrial unfolded protein response (UPRmt) as novel therapeutic targets for neurological disorders.
Chen X, An H, He J, Guo J, Xu S, Wu C, Wu D, Ji X. Chen X, et al. J Cereb Blood Flow Metab. 2025 May 15:271678X251341293. doi: 10.1177/0271678X251341293. Online ahead of print. J Cereb Blood Flow Metab. 2025. PMID: 40370320 Free PMC article. Review. - Proteostasis in striatal cells and selective neurodegeneration in Huntington's disease.
Margulis J, Finkbeiner S. Margulis J, et al. Front Cell Neurosci. 2014 Aug 7;8:218. doi: 10.3389/fncel.2014.00218. eCollection 2014. Front Cell Neurosci. 2014. PMID: 25147502 Free PMC article. Review. - Parkin deficiency modulates NLRP3 inflammasome activation by attenuating an A20-dependent negative feedback loop.
Mouton-Liger F, Rosazza T, Sepulveda-Diaz J, Ieang A, Hassoun SM, Claire E, Mangone G, Brice A, Michel PP, Corvol JC, Corti O. Mouton-Liger F, et al. Glia. 2018 Aug;66(8):1736-1751. doi: 10.1002/glia.23337. Epub 2018 Apr 17. Glia. 2018. PMID: 29665074 Free PMC article.
References
- Schapira AH, Cooper JM, Dexter D, Jenner P, Clark JB, et al. (1989) Mitochondrial complex I deficiency in Parkinson's disease. Lancet 1: 1269. - PubMed
- Mann VM, Cooper JM, Krige D, Daniel SE, Schapira AHV, et al. (1992) Brain, skeletal muscle and platelet homogenate mitochondrial function in Parkinson's disease. Brain 115: ?. - PubMed
- Cardoso SM (2011) The mitochondrial cascade hypothesis for Parkinson's disease. Curr Pharm Des 17: 3390–3397. - PubMed
- Corti O, Lesage S, Brice A (2011) What genetics tells us about the causes and mechanisms of Parkinson's disease. Physiol Rev 91: 1161–1218. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases