Bone marrow mesenchymal stromal cells drive protective M2 microglia polarization after brain trauma - PubMed (original) (raw)
doi: 10.1007/s13311-014-0277-y.
Francesca Pischiutta, Loredana Riganti, Federica Marchesi, Elena Turola, Stefano Fumagalli, Carlo Perego, Emanuela Parotto, Paola Vinci, Pietro Veglianese, Giovanna D'Amico, Claudia Verderio, Maria-Grazia De Simoni
Affiliations
- PMID: 24965140
- PMCID: PMC4121458
- DOI: 10.1007/s13311-014-0277-y
Bone marrow mesenchymal stromal cells drive protective M2 microglia polarization after brain trauma
Elisa R Zanier et al. Neurotherapeutics. 2014 Jul.
Abstract
Microglia/macrophages (M) are major contributors to postinjury inflammation, but they may also promote brain repair in response to specific environmental signals that drive classic (M1) or alternative (M2) polarization. We investigated the activation and functional changes of M in mice with traumatic brain injuries and receiving intracerebroventricular human bone marrow mesenchymal stromal cells (MSCs) or saline infusion. MSCs upregulated Ym1 and Arginase-1 mRNA (p < 0.001), two M2 markers of protective M polarization, at 3 and 7 d postinjury, and increased the number of Ym1(+) cells at 7 d postinjury (p < 0.05). MSCs reduced the presence of the lysosomal activity marker CD68 on the membrane surface of CD11b-positive M (p < 0.05), indicating reduced phagocytosis. MSC-mediated induction of the M2 phenotype in M was associated with early and persistent recovery of neurological functions evaluated up to 35 days postinjury (p < 0.01) and reparative changes of the lesioned microenvironment. In vitro, MSCs directly counteracted the proinflammatory response of primary murine microglia stimulated by tumor necrosis factor-α + interleukin 17 or by tumor necrosis factor-α + interferon-γ and induced M2 proregenerative traits, as indicated by the downregulation of inducible nitric oxide synthase and upregulation of Ym1 and CD206 mRNA (p < 0.01). In conclusion, we found evidence that MSCs can drive the M transcriptional environment and induce the acquisition of an early, persistent M2-beneficial phenotype both in vivo and in vitro. Increased Ym1 expression together with reduced in vivo phagocytosis suggests M selection by MSCs towards the M2a subpopulation, which is involved in growth stimulation and tissue repair.
Figures
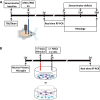
Fig. 1
Experimental design of in vivo and in vitro experiments. (A) In vivo experiments: traumatic brain injury (TBI)/sham surgery was done 1 d before treatment. Mesenchymal stromal cells (MSCs) or phosphate buffered saline (PBS) were infused in the contralateral ventricle. Sensorimotor deficits were evaluated at 0, 7, 21, and 35 d. Animals were sacrificed at 3 or 7 d for real time reverse transcription (RT) polymerase chain reaction (PCR) or at 7 or 35 d for histological analysis. (B) In vitro experiments: primary murine microglial cells were cultured for 48 h then, at the timepoints indicated in the plan, were exposed to 1) proinflammatory stimuli [tumor necrosis factor (TNF)-α/interleukin (IL-17) or TNF-α/interferon (IFN)-γ] for M1 classical activation; 2) MSCs; 3) proinflammatory stimuli followed by either direct or indirect (transwell) MSC co-culture. Unexposed cultures served as controls. After 72 h, co-cultures were analyzed by real-time PCR or immunohistochemistry. icv = intracerebroventricular
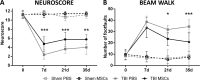
Fig. 2
Effects of infusion of mesenchymal stromal cells (MSCs) on sensorimotor deficits. Infusion of MSCs induced early and persistent improvement of sensorimotor deficits, as measured by (A) neuroscore or (B) beam walk tests. (A) Neuroscore test showed sensorimotor improvement in traumatic brain injury (TBI) MSCs mice from 7 d, while (B) the beam walk test showed a significant improvement of TBI MSCs from 21 d. Data are mean ± SD, n = 8, 2-way analysis of variance for RM followed by Tukey’s test. PBS = phosphate buffered saline
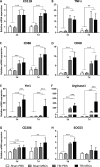
Fig. 3
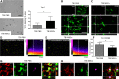
mRNA expression of genes related to microglia activation and polarization in brain cortices, 3 and 7d after surgery. (A–D) CD11b, TNFα, CD86, and CD68 were significantly upregulated in traumatic brain injury (TBI) mice compared with sham-operated mice both at 3 and 7 d after surgery, with no difference between TBI phosphate-buffered saline (PBS) and TBI mesenchymal stromal cells (MSCs) mice. (E–H) Ym1, Arginase-1, SOCS3, and CD206 were significantly upregulated in TBI mice compared with sham-operated mice at 3 d, but not at 7 d, after surgery. Infusion of MSCs significantly increased the expression of Ym1 and Arginase-1 in TBI MSCs mice compared with TBI PBS mice at both timepoints considered. No difference in the mRNA expression of SOCS3 was found between TBI PBS mice and TBI MSCs mice. At 7 d, TBI MSCs mice showed increased expression of CD206 compared with sham PBS mice. Data are expressed as the fold induction compared with the sham PBS group. Data are mean ± SD, n = 8. **p < 0.01, ***p < 0.001 versus sham or TBI PBS mice. Two-way analysis of variance followed by Tukey’s test
Fig. 4
Immunohistochemical analysis of CD45, CD11b, and CD68, and quantification of their co-localization in the injured cortex. Representative micrographs of (A) CD45, (B) CD11b, and (C) CD68 immunostainings and their quantifications 7 d after traumatic brain injury (TBI). The number of CD45high cells was significantly increased in TBI mesenchymal stromal cells (MSCs) mice, whereas no differences were observed in the expression of either CD11b or CD68. Representative micrographs of CD68 (green) and CD11b (red) co-localization in (D) TBI phosphate-buffered saline (PBS) and (D’) TBI MSCs mice. (D, merge) In TBI PBS mice, CD68 often co-localized with the membrane marker CD11b, while in TBI MSCs mice (D’, merge) it remained mainly located in the cytoplasm, thus yielding less co-localization with CD11b. (E, E’) Quantification of co-localized voxels in the 3-dimensional confocal acquisitions showed a reduction of CD68/CD11b-positive voxels after infusion of MSCs, indicating a reduction of lysosomal activity in TBI MSCs mice compared with TBI PBS mice (F). Data are mean ± SD, n = 8 (A, B, E). *p < 0.05, unpaired t test. Bars = 20 μm
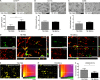
Fig. 5
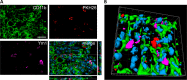
Immunohistochemical analysis of Ym1 in the injured cortex and quantification of its co-localization with CD68 in the injured hippocampus. Representative micrographs of (A) Ym1 immunostaining 7 d after traumatic brain injury (TBI) in phosphate-buffered saline (PBS)- or mesenchymal stromal cells (MSCs) treated mice, and its related quantification, which shows an increase in the expression of Ym1 in TBI MSCs mice compared with TBI PBS mice. Co-localization of Ym1 (purple) and CD68 (green) in (B) TBI PBS and (C) TBI MSCs mice. Bar = 20 μm. (D, E) Quantification of co-localized voxels in the 3-dimensional (3D) confocal acquisitions showed a reduction of (F) Ym1/CD68-positive voxels after infusion of MSCs. Triple immunofluorescence for CD11b (red), CD68 (green), and Ym1 (purple) for (G) TBI PBS and (H) TBS MSCs mice. (G) In TBI PBS mice, co-localization between Ym1 and CD68 (white) is observed in cells with strong co-localization between CD68 and CD11b (yellow). Xyz-view and 3D renderings of co-localized pixels (centre and right panels) better illustrate this. (H) In TBI MSCs mice, cells with reduced Ym1/CD68 co-localization (white) show diminished CD68/CD11b co-localization. The Xyz-view and 3D renderings of co-localized pixels are shown in centre and right panels in (H). Bar = 5 μm. Data are mean ± SD, n = 8 (A, F). *p < 0.05, unpaired t test
Fig. 6
Localization of CD11b/Ym1 double-positive cells with PKH26-labeled mesenchymal stromal cells (MSCs) in the injured cortex. (A) At 7 d after traumatic brain injury, in mice infused with MSCs, the cells positive for CD11b (green) and Ym1 (purple) showed direct contact with infused MSCs (PKH26, red, A), as better depicted in (B) the 3-dimensional rendering. Bar = 20 μm
Fig. 7
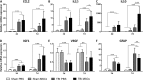
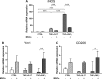
mRNA expression of cytokines and growth factors in brain cortices, 3 and 7 d after surgery. (A–D) CCL2, IL-1β, IL-10, and IGF1 were significantly upregulated in traumatic brain injury (TBI) compared with sham-operated mice at 3 d after surgery. (A–B–D) The upregulation of CCL2, IL-1β, and IGF1 in TBI mice persisted at 7 d after surgery. (A–D) There was no difference in the expression of CCL2, IL-1β, IL-10, and IGF1 between TBI phosphate buffered saline (PBS) and TBI MSCs mice at 3 d. (A–C) At 7 d, mesenchymal stromal cells (MSCs) infusion significantly increased the expression of CCL2, IL-1β, and IL-10 in TBI MSCs mice compared with TBI PBS mice, while (D) no difference was found in the expression of IGF1. (E) The expression of VEGF was downregulated in TBI PBS mice compared with sham-operated groups both at 3 d and 7 d. TBI MSCs mice showed a trend toward an increase in the expression of VEGF at 7d, restoring VEGF expression close to the levels observed in sham-operated animals. (F) The expression of GFAP was significantly upregulated in TBI mice compared with sham-operated mice at both 3 and 7 d after surgery. Infusion of MSCs induced a significant reduction in the expression of GFAP at 7 d after surgery. Data are shown as fold induction compared with sham PBS group and are mean ± SD, n = 8. **p < 0.01, ***p < 0.001 versus sham or TBI PBS, 2-way analysis of variance followed by Tukey’s test
Fig. 8
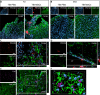
Localization of PKH26-labeled mesenchymal stromal cells (MSCs) and immunofluorescence for growth associated protein 43 (GAP-43) and Ym1 in the injured cortex. Representative micrographs at low magnification showing the presence of PKH26-labeled MSCs (red) in cortical areas positive for GAP-43 (green) at (A) 7 d or (B) 35 d after traumatic brain injury (TBI; nuclei are in blue, bar = 100 μm). PKH26 was visible only at 7 d in mice infused with MSCs, while no PKH26 positivity was detectable at 35 d in either phosphate-buffered saline (PBS)- or MSC-treated mice. GAP-43 appeared to be increased at 7 and 35 d in mice receiving MSCs. (C) At 7 d, in TBI MSCs mice, PKH26-positive cells were found either in association with GAP-43-positive cells or in areas negative for GAP-43. (D) When MSCs reached the neurogenic niche in the subventricular zone, they localized close to GAP-43-positive cells. (E) M2 polarized cells (Ym1-positive, purple) were located in areas positive for GAP-43 and showed strong association with GAP-43-positive cells [(F) 3-dimensional rendering]. Bars in (C–E) = 20 μm. DAPI = 4’6-diamidino-2-phenylindole; LV = lateral ventricle
Fig. 9
In vitro analysis of microglia markers after exposure to mesenchymal stromal cells (MSCs). Microglia expression of (A, B) Ym1 and (C, D) CD206 in control conditions or in co-culture with MSCs for 72 h. (A, C) Quantification of mRNA expression indicates an upregulation of both M2 markers induced by MSC co-culture. Representative confocal images of pure microglial cultures and microglia co-cultured with MSCs stained for (B) Ym1 and (D) CD206. Data are mean ± SD from three independent experiments.*p < 0.05, **p < 0.01, unpaired t test. CTRL = control
Fig. 10
In vitro analysis of M1 and M2 polarization markers following exposure to proinflammatory stimuli and mesenchymal stromal cells (MSCs). mRNA expression for (A) iNOS, (B) Ym1, and (C) CD206 in control or activated [exposed to tumor necrosis factor (TNF)-α/interleukin (IL)-17 or TNF-α/interferon (IFN)-γ] microglia, maintained in vitro in isolation or co-cultured with MSCs. Data are mean ± SD from 3 independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001 2-way analysis of variance followed by Tukey’s test
Similar articles
- Heterogeneous induction of microglia M2a phenotype by central administration of interleukin-4.
Pepe G, Calderazzi G, De Maglie M, Villa AM, Vegeto E. Pepe G, et al. J Neuroinflammation. 2014 Dec 31;11:211. doi: 10.1186/s12974-014-0211-6. J Neuroinflammation. 2014. PMID: 25551794 Free PMC article. - Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages.
Cho DI, Kim MR, Jeong HY, Jeong HC, Jeong MH, Yoon SH, Kim YS, Ahn Y. Cho DI, et al. Exp Mol Med. 2014 Jan 10;46(1):e70. doi: 10.1038/emm.2013.135. Exp Mol Med. 2014. PMID: 24406319 Free PMC article. - Macrophages are essential for maintaining a M2 protective response early after ischemic brain injury.
Perego C, Fumagalli S, Zanier ER, Carlino E, Panini N, Erba E, De Simoni MG. Perego C, et al. Neurobiol Dis. 2016 Dec;96:284-293. doi: 10.1016/j.nbd.2016.09.017. Epub 2016 Sep 30. Neurobiol Dis. 2016. PMID: 27697537 - Mesenchymal stem cell-based treatments for stroke, neural trauma, and heat stroke.
Hsuan YC, Lin CH, Chang CP, Lin MT. Hsuan YC, et al. Brain Behav. 2016 Aug 3;6(10):e00526. doi: 10.1002/brb3.526. eCollection 2016 Oct. Brain Behav. 2016. PMID: 27781140 Free PMC article. Review. - Mesenchymal stem cells maintain the microenvironment of central nervous system by regulating the polarization of macrophages/microglia after traumatic brain injury.
Xu C, Fu F, Li X, Zhang S. Xu C, et al. Int J Neurosci. 2017 Dec;127(12):1124-1135. doi: 10.1080/00207454.2017.1325884. Epub 2017 May 19. Int J Neurosci. 2017. PMID: 28464695 Review.
Cited by
- MSCs and Inflammatory Cells Crosstalk in Regenerative Medicine: Concerted Actions for Optimized Resolution Driven by Energy Metabolism.
Planat-Benard V, Varin A, Casteilla L. Planat-Benard V, et al. Front Immunol. 2021 Apr 30;12:626755. doi: 10.3389/fimmu.2021.626755. eCollection 2021. Front Immunol. 2021. PMID: 33995350 Free PMC article. Review. - Long pentraxin PTX3 is upregulated systemically and centrally after experimental neurotrauma, but its depletion leaves unaltered sensorimotor deficits or histopathology.
Oggioni M, Mercurio D, Minuta D, Fumagalli S, Popiolek-Barczyk K, Sironi M, Ciechanowska A, Ippati S, De Blasio D, Perego C, Mika J, Garlanda C, De Simoni MG. Oggioni M, et al. Sci Rep. 2021 May 5;11(1):9616. doi: 10.1038/s41598-021-89032-7. Sci Rep. 2021. PMID: 33953334 Free PMC article. - Repair of the Injured Spinal Cord by Schwann Cell Transplantation.
Fu H, Hu D, Chen J, Wang Q, Zhang Y, Qi C, Yu T. Fu H, et al. Front Neurosci. 2022 Feb 17;16:800513. doi: 10.3389/fnins.2022.800513. eCollection 2022. Front Neurosci. 2022. PMID: 35250447 Free PMC article. Review. - The phagocytic state of brain myeloid cells after ischemia revealed by superresolution structured illumination microscopy.
Fumagalli S, Fiordaliso F, Perego C, Corbelli A, Mariani A, De Paola M, De Simoni MG. Fumagalli S, et al. J Neuroinflammation. 2019 Jan 16;16(1):9. doi: 10.1186/s12974-019-1401-z. J Neuroinflammation. 2019. PMID: 30651101 Free PMC article. - Neuroprotection in Traumatic Brain Injury: Mesenchymal Stromal Cells can Potentially Overcome Some Limitations of Previous Clinical Trials.
Carbonara M, Fossi F, Zoerle T, Ortolano F, Moro F, Pischiutta F, Zanier ER, Stocchetti N. Carbonara M, et al. Front Neurol. 2018 Oct 24;9:885. doi: 10.3389/fneur.2018.00885. eCollection 2018. Front Neurol. 2018. PMID: 30405517 Free PMC article. Review.
References
- Xiong Y, Mahmood A, Meng Y, et al. Delayed administration of erythropoietin reducing hippocampal cell loss, enhancing angiogenesis and neurogenesis, and improving functional outcome following traumatic brain injury in rats: comparison of treatment with single and triple dose. J Neurosurg. 2010;113:598–608. doi: 10.3171/2009.9.JNS09844. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous