Circulating microRNA signature in non-alcoholic fatty liver disease: from serum non-coding RNAs to liver histology and disease pathogenesis - PubMed (original) (raw)
Circulating microRNA signature in non-alcoholic fatty liver disease: from serum non-coding RNAs to liver histology and disease pathogenesis
Carlos J Pirola et al. Gut. 2015 May.
Abstract
Objectives: We used a screening strategy of global serum microRNA (miRNA) profiling, followed by a second stage of independent replication and exploration of liver expression of selected miRNAs to study: (1) the circulating miRNA signature associated with non-alcoholic fatty liver disease (NAFLD) progression and predictive power, (2) the role of miRNAs in disease biology and (3) the association between circulating miRNAs and features of the metabolic syndrome.
Methods: The study used a case-control design and included patients with NAFLD proven through biopsy and healthy controls.
Results: Among 84 circulating miRNAs analysed, miR-122, miR-192, miR-19a and miR-19b, miR-125b, and miR-375 were upregulated >2-fold (p<0.05) either in simple steatosis (SS) or non-alcoholic steatohepatitis (NASH). The most dramatic and significant fold changes were observed in the serum levels of miR-122 (7.2-fold change in NASH vs controls and 3.1-fold change in NASH vs SS) and miR-192 (4.4-fold change in NASH vs controls); these results were replicated in the validation set. The majority of serum miR-122 circulate in argonaute2-free forms. Circulating miR-19a/b and miR-125b were correlated with biomarkers of atherosclerosis. Liver miR-122 expression was 10-fold (p<0.03) downregulated in NASH compared with SS and was preferentially expressed at the edge of lipid-laden hepatocytes. In vitro exploration showed that overexpression of miR-122 enhances alanine aminotransferase activity.
Conclusions: miR-122 plays a role of physiological significance in the biology of NAFLD; circulating miRNAs mirror the histological and molecular events occurring in the liver. NAFLD has a distinguishing circulating miRNA profile associated with a global dysmetabolic disease state and cardiovascular risk.
Keywords: Liver; Nonalcoholic Steatohepatitis.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Conflict of interest statement
Competing interests TFG, MMGL, DF, SS and CJP belong to the Consejo Nacional de Investigaciones Científicas y Tecnologicas (CONICET). AJS was also supported by a grant from the NIH RO1 DK 081410. CJP, TGF, GOC, PM, JSM, MMGLL, DF, FM, AJS and SS have no direct competing interests to declare.
Figures
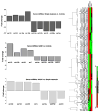
Figure 1
Global circulating miRNA expression profile in non-alcoholic fatty liver disease (NAFLD) patients and control subjects. Left, a heat map shows the results of 84 differentially expressed miRNAs in NAFLD patients compared with healthy controls. Red, overexpressed miRNAs; green, downregulated miRNAs; black, no change. The three groups of samples: control group, group 1 (simple steatosis) and group 2 (non-alcoholic steatohepatitis), can be distinguished according to the profile. Right, bars show the fold changes of significantly dysregulated miRNAs (p<0.05) according to disease severity.
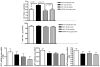
Figure 2
Validation study of selected circulating miRNAs in non-alcoholic fatty liver disease (NAFLD) patients and control subjects, and diagnostic accuracy in predicting histological disease severity. Circulating levels of miR-122, miR-192 and miR-375 are expressed relative to miR-23a. NASH, non-alcoholic steatohepatitis; SS, simple steatosis. The criteria for evaluating the severity of fatty liver infiltration, NAS and hepatocellular ballooning are described in the Methods Section. The receiver operating characteristic (ROC) curves with the corresponding area under the ROC curves for different histological features in NAFLD patients are shown.
Figure 3
Comparison of the diagnostic accuracy of circulating miR-122 and classic serum biomarkers in predicting disease severity. Receiver operating characteristic (ROC) curves with corresponding area under the ROC curves for comparing the ability of miR-122 and serum disease biomarkers, such as liver enzymes (serum alanine and aspartate aminotransferase (ALT and AST)) and caspase-generated CK-18 fragments, to distinguish the severity of non-alcoholic fatty liver disease (non-alcoholic steatohepatitis and liver fibrosis) are shown.
Figure 4
Analysis of circulating miR-122 in supernatant and miR-122 in argonaute2 (Ago2)-immunoprecipitated complexes in patients with non-alcoholic fatty liver disease (NAFLD). (A) Measurement of circulating expression of miR-122 in serum of controls and NAFLD patients after immunoprecipitation of serum with Ago2 (miR-122 in supernatant/miR-122 coimmunoprecipitated with anti-Ago2 antibody). (B) The scores on liver protein expression of Ago2 evaluated by immunohistochemistry with the use of a monoclonal antihuman antibody according to disease status. Results are expressed as mean±SE and median (range). The p value stands for statistical significance in a Kruskal–Wallis test. Bottom, a representative liver expression pattern of Ago2 evaluated by immunohistochemistry in patients with NAFLD and in control liver (black arrowheads show positive staining). Ago2 immunoreactivity was examined by light microscopy of liver sections. Anti-Ago2 antibody immunostaining was significantly observed in the cytoplasm of hepatocytes and was primarily but not exclusively restricted to lipid-laden ones (inset: fat droplet, FD) and the edge of the wall of hepatocytes (inset) showing a granular pattern. Lobular inflammatory infiltrate showed negative immunoreactivity to Ago2. Counterstaining was done with haematoxylin. Original magnification: 400×.
Figure 5
Liver expression of candidate miRNAs and localisation by in situ hybridisation (ISH). Right, liver expression of miR-122 (A) and miR-192 (B) in non-alcoholic fatty liver disease (NAFLD) patients and subjects with near-normal liver histology. Left, a representative liver miR-122 ISH analysis in cases and controls, showing positive staining in blue/purple and nuclei staining in pink. Overall, the staining was particularly localised around the lipid droplets (black arrows), regardless of the NAFLD histological severity, and at the edge of the wall of hepatocytes. Original magnification: 400×.
Figure 6
In vitro exploration of the effect of miR-122 on alanine (ALT) and aspartate aminotransaminases (AST). Huh7 human hepatoma cells were transfected with a synthetic miR-122 and a miR-122 inhibitor to explore the role of miR-122 in the liver messenger RNA (mRNA) expression and enzymatic activity of liver transaminases. The liver expression of GPT1, GOT1 and GOT2 mRNA was unchanged during both miR-122 overexpression and silencing, whereas the ALT (GPT) enzymatic activity in cell lysates showed a significant increase when cells were transfected with the miR-122 mimic. GPT1, cytosolic alanine aminotransaminase 1; GOT1, glutamic-oxaloacetic transaminase 1, soluble; GOT2, glutamic-oxaloacetic transaminase 2, mitochondrial.
Similar articles
- Disease-specific miR-34a as diagnostic marker of non-alcoholic steatohepatitis in a Chinese population.
Liu XL, Pan Q, Zhang RN, Shen F, Yan SY, Sun C, Xu ZJ, Chen YW, Fan JG. Liu XL, et al. World J Gastroenterol. 2016 Nov 28;22(44):9844-9852. doi: 10.3748/wjg.v22.i44.9844. World J Gastroenterol. 2016. PMID: 27956809 Free PMC article. - Increased serum miR-193a-5p during non-alcoholic fatty liver disease progression: Diagnostic and mechanistic relevance.
Johnson K, Leary PJ, Govaere O, Barter MJ, Charlton SH, Cockell SJ, Tiniakos D, Zatorska M, Bedossa P, Brosnan MJ, Cobbold JF, Ekstedt M, Aithal GP, Clément K, Schattenberg JM, Boursier J, Ratziu V, Bugianesi E, Anstee QM, Daly AK; LITMUS Consortium Investigators§; LITMUS Consortium Investigators. Johnson K, et al. JHEP Rep. 2021 Nov 25;4(2):100409. doi: 10.1016/j.jhepr.2021.100409. eCollection 2022 Feb. JHEP Rep. 2021. PMID: 35072021 Free PMC article. - Serum coding and non-coding RNAs as biomarkers of NAFLD and fibrosis severity.
Di Mauro S, Scamporrino A, Petta S, Urbano F, Filippello A, Ragusa M, Di Martino MT, Scionti F, Grimaudo S, Pipitone RM, Privitera G, Di Pino A, Scicali R, Valenti L, Dongiovanni P, Fracanzani A, Rabuazzo AM, Craxì A, Purrello M, Purrello F, Piro S. Di Mauro S, et al. Liver Int. 2019 Sep;39(9):1742-1754. doi: 10.1111/liv.14167. Epub 2019 Jun 26. Liver Int. 2019. PMID: 31169972 Free PMC article. - Circulating miRNAs associated with nonalcoholic fatty liver disease.
Atic AI, Thiele M, Munk A, Dalgaard LT. Atic AI, et al. Am J Physiol Cell Physiol. 2023 Feb 1;324(2):C588-C602. doi: 10.1152/ajpcell.00253.2022. Epub 2023 Jan 16. Am J Physiol Cell Physiol. 2023. PMID: 36645666 Review. - MicroRNAs in non-alcoholic fatty liver disease: Progress and perspectives.
Hochreuter MY, Dall M, Treebak JT, Barrès R. Hochreuter MY, et al. Mol Metab. 2022 Nov;65:101581. doi: 10.1016/j.molmet.2022.101581. Epub 2022 Aug 23. Mol Metab. 2022. PMID: 36028120 Free PMC article. Review.
Cited by
- Moringa oleifera Improves MAFLD by Inducing Epigenetic Modifications.
Monraz-Méndez CA, Escutia-Gutiérrez R, Rodriguez-Sanabria JS, Galicia-Moreno M, Monroy-Ramírez HC, Sánchez-Orozco L, García-Bañuelos J, De la Rosa-Bibiano R, Santos A, Armendáriz-Borunda J, Sandoval-Rodríguez A. Monraz-Méndez CA, et al. Nutrients. 2022 Oct 11;14(20):4225. doi: 10.3390/nu14204225. Nutrients. 2022. PMID: 36296907 Free PMC article. - Liver microRNAs: potential mediators and biomarkers for metabolic and cardiovascular disease?
Willeit P, Skroblin P, Kiechl S, Fernández-Hernando C, Mayr M. Willeit P, et al. Eur Heart J. 2016 Nov 14;37(43):3260-3266. doi: 10.1093/eurheartj/ehw146. Epub 2016 Apr 20. Eur Heart J. 2016. PMID: 27099265 Free PMC article. Review. - Advances in the Diagnosis and Treatment of Non-Alcoholic Fatty Liver Disease.
Yin X, Guo X, Liu Z, Wang J. Yin X, et al. Int J Mol Sci. 2023 Feb 2;24(3):2844. doi: 10.3390/ijms24032844. Int J Mol Sci. 2023. PMID: 36769165 Free PMC article. Review. - Circulating miRNAs as Novel Diagnostic Biomarkers in Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis.
Cai C, Lin Y, Yu C. Cai C, et al. Can J Gastroenterol Hepatol. 2019 Aug 20;2019:2096161. doi: 10.1155/2019/2096161. eCollection 2019. Can J Gastroenterol Hepatol. 2019. PMID: 31531307 Free PMC article. - NAFLD: Mechanisms, Treatments, and Biomarkers.
Nassir F. Nassir F. Biomolecules. 2022 Jun 13;12(6):824. doi: 10.3390/biom12060824. Biomolecules. 2022. PMID: 35740949 Free PMC article. Review.
References
- Selbach M, Schwanhausser B, Thierfelder N, et al. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455:58–63. - PubMed
- Weiland M, Gao XH, Zhou L, et al. Small RNAs have a large impact: circulating microRNAs as biomarkers for human diseases. RNA Biol. 2012;9:850–9. - PubMed
- Creemers EE, Tijsen AJ, Pinto YM. Circulating microRNAs: novel biomarkers and extracellular communicators in cardiovascular disease? Circ Res. 2012;110:483–95. - PubMed
- Pirola CJ, Gianotti TF, Castano GO, et al. Circulating MicroRNA-122 signature in nonalcoholic fatty liver disease and cardiovascular disease: a new endocrine system in metabolic syndrome. Hepatology. 2013;57:2545–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical