Safely targeting cancer stem cells via selective catenin coactivator antagonism - PubMed (original) (raw)
Review
. 2014 Sep;105(9):1087-92.
doi: 10.1111/cas.12471. Epub 2014 Sep 6.
Affiliations
- PMID: 24975284
- PMCID: PMC4175086
- DOI: 10.1111/cas.12471
Review
Safely targeting cancer stem cells via selective catenin coactivator antagonism
Heinz-Josef Lenz et al. Cancer Sci. 2014 Sep.
Abstract
Throughout our life, long-lived somatic stem cells (SSC) regenerate adult tissues both during homeostatic processes and repair after injury. The role of aberrant regulation of SSC has also recently gained prominence in the field of cancer research. Following malignant transformation, so termed cancer stem cells (CSC), endowed with the same properties as SSC (i.e. the ability to both self-renew and generate differentiated progenitors), play a major part in tumor initiation, therapy resistance and ultimately relapse. The same signaling pathways involved in regulating SSC maintenance are involved in the regulation of CSC. CSC exist in a wide array of tumor types, including leukemias, and brain, breast, prostate and colon tumors. Consequently, one of the key goals in cancer research over the past decade has been to develop therapeutic strategies to safely eliminate the CSC population without damaging the endogenous SSC population. A major hurdle to this goal lies in the identification of the key mechanisms that distinguish CSC from the normal endogenous tissue stem cells. This review will discuss the discovery of the specific CBP/catenin antagonist ICG-001 and the ongoing clinical development of the second generation CBP/catenin antagonist PRI-724. Importantly, specific CBP/catenin antagonists appear to have the ability to safely eliminate CSC by taking advantage of an intrinsic differential preference in the way SSC and CSC divide.
Keywords: Asymmetric; CREB-binding protein; Wnt/catenin; p300; symmetric.
© 2014 The Authors. Cancer Science published by Wiley Publishing Asia Pty Ltd on behalf of Japanese Cancer Association.
Figures
Fig 1
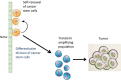
Cancer stem cells both self-renew and undergo differentiative divisions to maintain or expand the cancer stem cell population or generate transient amplifying cells that go on to rapidly divide to form the bulk of the tumor.
Fig 2
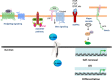
The ultimate decision for a cell to retain potency or initiate differentiation is dependent upon numerous inputs from various signaling pathways (e.g. JAK/STAT, Wnt, Growth Factors, Hippo, Notch and Hedgehog) that also play critical roles during development. In the end, those multiple pathways must be integrated and funneled down into a simple decision point. β-catenin plays a central role in integrating these signals.
Fig 3
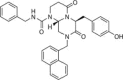
Chemical structure of the CBP/catenin antagonist ICG-001.
Fig 4
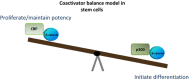
Wnt signaling is a complex pathway, believed to be involved in the regulation of divergent processes, including the maintenance of pluripotency and commitment to differentiation. We developed a model in which β-catenin/CBP-mediated transcription is critical for the maintenance of potency, whereas β-catenin/p300-mediated transcription is the first critical step to initiate differentiation. Hence, the balance between CBP and p300-mediated β-catenin transcription regulates the balance between maintenance of potency, and the initiation of commitment to differentiate in stem and progenitor cells.
Fig 5
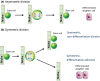
Model depicting symmetric and asymmetric modes of division. The intrinsic difference between normal somatic stem cells (SSC) and cancer stem cells (CSC) is that normal SSC favor asymmetric division whereas CSC favor symmetric divisions. Treatment of CSC with CBP/catenin antagonists causes CSC to undergo symmetric differentiative divisions, thereby eventually clearing CSC from the niche. In sharp contrast, SSC undergo asymmetric divisions when treated with CBP/catenin antagonists.
Similar articles
- Small-molecule inhibition of CBP/catenin interactions eliminates drug-resistant clones in acute lymphoblastic leukemia.
Gang EJ, Hsieh YT, Pham J, Zhao Y, Nguyen C, Huantes S, Park E, Naing K, Klemm L, Swaminathan S, Conway EM, Pelus LM, Crispino J, Mullighan CG, McMillan M, Müschen M, Kahn M, Kim YM. Gang EJ, et al. Oncogene. 2014 Apr 24;33(17):2169-78. doi: 10.1038/onc.2013.169. Epub 2013 Jun 3. Oncogene. 2014. PMID: 23728349 Free PMC article. - Sam68 Allows Selective Targeting of Human Cancer Stem Cells.
Benoit YD, Mitchell RR, Risueño RM, Orlando L, Tanasijevic B, Boyd AL, Aslostovar L, Salci KR, Shapovalova Z, Russell J, Eguchi M, Golubeva D, Graham M, Xenocostas A, Trus MR, Foley R, Leber B, Collins TJ, Bhatia M. Benoit YD, et al. Cell Chem Biol. 2017 Jul 20;24(7):833-844.e9. doi: 10.1016/j.chembiol.2017.05.026. Epub 2017 Jun 22. Cell Chem Biol. 2017. PMID: 28648376 - CBP/Catenin antagonists: Targeting LSCs' Achilles heel.
Kim YM, Gang EJ, Kahn M. Kim YM, et al. Exp Hematol. 2017 Aug;52:1-11. doi: 10.1016/j.exphem.2017.04.010. Epub 2017 May 4. Exp Hematol. 2017. PMID: 28479420 Free PMC article. Review. - Functional and genomic analyses reveal therapeutic potential of targeting β-catenin/CBP activity in head and neck cancer.
Kartha VK, Alamoud KA, Sadykov K, Nguyen BC, Laroche F, Feng H, Lee J, Pai SI, Varelas X, Egloff AM, Snyder-Cappione JE, Belkina AC, Bais MV, Monti S, Kukuruzinska MA. Kartha VK, et al. Genome Med. 2018 Jul 20;10(1):54. doi: 10.1186/s13073-018-0569-7. Genome Med. 2018. PMID: 30029671 Free PMC article. - Kat3 coactivators in somatic stem cells and cancer stem cells: biological roles, evolution, and pharmacologic manipulation.
Thomas PD, Kahn M. Thomas PD, et al. Cell Biol Toxicol. 2016 Feb;32(1):61-81. doi: 10.1007/s10565-016-9318-0. Epub 2016 Mar 23. Cell Biol Toxicol. 2016. PMID: 27008332 Free PMC article. Review.
Cited by
- A Genome-Wide RNA Interference Screen Identifies a Role for Wnt/β-Catenin Signaling during Rift Valley Fever Virus Infection.
Harmon B, Bird SW, Schudel BR, Hatch AV, Rasley A, Negrete OA. Harmon B, et al. J Virol. 2016 Jul 27;90(16):7084-7097. doi: 10.1128/JVI.00543-16. Print 2016 Aug 15. J Virol. 2016. PMID: 27226375 Free PMC article. - Novel targeted therapies in adrenocortical carcinoma.
Konda B, Kirschner LS. Konda B, et al. Curr Opin Endocrinol Diabetes Obes. 2016 Jun;23(3):233-41. doi: 10.1097/MED.0000000000000247. Curr Opin Endocrinol Diabetes Obes. 2016. PMID: 27119750 Free PMC article. Review. - Pharmacological Modulation of the Wnt/β-Catenin Pathway Inhibits Proliferation and Promotes Differentiation of Long-Lived Memory CD4+ T Cells in Antiretroviral Therapy-Suppressed Simian Immunodeficiency Virus-Infected Macaques.
Mavigner M, Zanoni M, Tharp GK, Habib J, Mattingly CR, Lichterfeld M, Nega MT, Vanderford TH, Bosinger SE, Chahroudi A. Mavigner M, et al. J Virol. 2019 Dec 12;94(1):e01094-19. doi: 10.1128/JVI.01094-19. Print 2019 Dec 12. J Virol. 2019. PMID: 31619550 Free PMC article. - Inhibition of CBP/β-catenin and porcupine attenuates Wnt signaling and induces apoptosis in head and neck carcinoma cells.
Kleszcz R, Szymańska A, Krajka-Kuźniak V, Baer-Dubowska W, Paluszczak J. Kleszcz R, et al. Cell Oncol (Dordr). 2019 Aug;42(4):505-520. doi: 10.1007/s13402-019-00440-4. Epub 2019 May 14. Cell Oncol (Dordr). 2019. PMID: 31089983 - Inhibition of Wnt/beta-catenin signaling downregulates expression of aldehyde dehydrogenase isoform 3A1 (ALDH3A1) to reduce resistance against temozolomide in glioblastoma in vitro.
Suwala AK, Koch K, Rios DH, Aretz P, Uhlmann C, Ogorek I, Felsberg J, Reifenberger G, Köhrer K, Deenen R, Steiger HJ, Kahlert UD, Maciaczyk J. Suwala AK, et al. Oncotarget. 2018 Apr 27;9(32):22703-22716. doi: 10.18632/oncotarget.25210. eCollection 2018 Apr 27. Oncotarget. 2018. PMID: 29854309 Free PMC article.
References
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. - PubMed
- Singh SR. Stem cell niche in tissue homeostasis, aging and cancer. Curr Med Chem. 2012;19:5965–74. - PubMed
- Miki T, Yasuda SY, Kahn M. “Wnt/Β-catenin signaling in embryonic stem cell self-renewal and somatic cell reprogramming. Stem Cell Rev. 2011;7:836–46. - PubMed
- Takahashi-Yanaga F, Kahn M. Targeting Wnt signaling: can we safely eradicate cancer stem cells? Clin Cancer Res. 2010;16:3153–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA166161/CA/NCI NIH HHS/United States
- 1R01 HL112638-01/HL/NHLBI NIH HHS/United States
- 1R01CA166161-01A1/CA/NCI NIH HHS/United States
- 1R21AI105057-01/AI/NIAID NIH HHS/United States
- P30 CA014089/CA/NCI NIH HHS/United States
- 1R21NS074392-01/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous