Redox imbalance and morphological changes in skin fibroblasts in typical Rett syndrome - PubMed (original) (raw)
doi: 10.1155/2014/195935. Epub 2014 May 29.
Silvia Leoncini 2, Claudio De Felice [ 3](#full-view-affiliation-3 "Neonatal Intensive Care Unit, University Hospital AOUS, Policlinico "S. M. alle Scotte," 53100 Siena, Italy."), Alessandra Pecorelli 2, Ilaria Meloni 4, Francesca Ariani 4, Francesca Mari 4, Sonia Amabile 4, Eugenio Paccagnini 5, Mariangela Gentile 5, Giuseppe Belmonte 6, Gloria Zollo 2, Giuseppe Valacchi 7, Thierry Durand 8, Jean-Marie Galano 8, Lucia Ciccoli 1, Alessandra Renieri 9, Joussef Hayek 10
Affiliations
- PMID: 24987493
- PMCID: PMC4060159
- DOI: 10.1155/2014/195935
Redox imbalance and morphological changes in skin fibroblasts in typical Rett syndrome
Cinzia Signorini et al. Oxid Med Cell Longev. 2014.
Abstract
Evidence of oxidative stress has been reported in the blood of patients with Rett syndrome (RTT), a neurodevelopmental disorder mainly caused by mutations in the gene encoding the Methyl-CpG-binding protein 2. Little is known regarding the redox status in RTT cellular systems and its relationship with the morphological phenotype. In RTT patients (n = 16) we investigated four different oxidative stress markers, F2-Isoprostanes (F2-IsoPs), F4-Neuroprostanes (F4-NeuroPs), nonprotein bound iron (NPBI), and (4-HNE PAs), and glutathione in one of the most accessible cells, that is, skin fibroblasts, and searched for possible changes in cellular/intracellular structure and qualitative modifications of synthesized collagen. Significantly increased F4-NeuroPs (12-folds), F2-IsoPs (7.5-folds) NPBI (2.3-folds), 4-HNE PAs (1.48-folds), and GSSG (1.44-folds) were detected, with significantly decreased GSH (-43.6%) and GSH/GSSG ratio (-3.05 folds). A marked dilation of the rough endoplasmic reticulum cisternae, associated with several cytoplasmic multilamellar bodies, was detectable in RTT fibroblasts. Colocalization of collagen I and collagen III, as well as the percentage of type I collagen as derived by semiquantitative immunofluorescence staining analyses, appears to be significantly reduced in RTT cells. Our findings indicate the presence of a redox imbalance and previously unrecognized morphological skin fibroblast abnormalities in RTT patients.
Figures
Figure 1
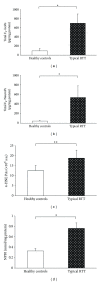
Increased levels of total (i.e., sum of free and esterified form) F2-IsoPs, total F4-NeuroPs, 4-HNE PAs, and NPBI in RTT skin fibroblast as compared to the control cells. *P < 0.0001, **P = 0.0013. Data are expressed as means ± standard deviation. Legend: F2-IsoPs, F2-isoprostanes; F4-NeuroPs, F4-neuroprostanes; 4-HNE PAs, 4-hydroxy-2-nonenal protein adducts; NPBI, nonprotein bound iron.
Figure 2
Significant reduction in cellular GSH and significant increase of GSSG in RTT skin fibroblast as compared to control cells. *P < 0.0001, **P = 0.0033. Data are expressed as means ± standard deviation. Legend: GSH reduced glutathione; GSSG, oxidized glutathione.
Figure 3
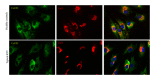
Transmission electron microscopy of control (a) and RTT (b) fibroblasts cultures. Skin fibroblasts, either from control subjects or RTT patients, show a flattened morphology with extensive tapering cytoplasmic processes. An euchromatic and oval-shaped nucleus was present in central position of the cells, with clumps of heterochromatin next to the nuclear envelope. The cytoplasm contains many vesicles with variable electron density, a prominent Golgi complex, and mitochondria. Rough endoplasmic reticulum (RER) cisternae in RTT fibroblasts appear more dilated than in control. Some large multilamellar bodies (MLB) are frequently detectable in the cytoplasm of the RTT fibroblast cells. (G) Golgi complex, (M) mitochondrion, and (V) vesicle. Bar = 1 _μ_m.
Figure 4
Double immunofluorescence staining shows the localization of type I collagen (central column, red color) and type III collagen (left column, green color). Images are merged in the right panel and the yellow color indicates overlap of the staining. The colocalization of types I and III collagen is reduced in RTT skin fibroblasts. Legend: Col I, type I collagen; Col III, type III collagen.
Figure 5
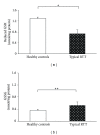
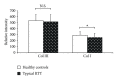
Relative intensity of fluorescence for types I and type III collagen in RTT and control skin fibroblasts. Software LEICA AF6000 (Leica Microsystems-Germany). Data are expressed as median ± semiinterquartile range *P = 0.0062; N.S.: no significant difference (P = 0.4361). Legend: Col I, type I collagen; Col III, type III collagen.
Similar articles
- Inflammatory lung disease in Rett syndrome.
De Felice C, Rossi M, Leoncini S, Chisci G, Signorini C, Lonetti G, Vannuccini L, Spina D, Ginori A, Iacona I, Cortelazzo A, Pecorelli A, Valacchi G, Ciccoli L, Pizzorusso T, Hayek J. De Felice C, et al. Mediators Inflamm. 2014;2014:560120. doi: 10.1155/2014/560120. Epub 2014 Mar 17. Mediators Inflamm. 2014. PMID: 24757286 Free PMC article. - Morphological changes and oxidative damage in Rett Syndrome erythrocytes.
Ciccoli L, De Felice C, Paccagnini E, Leoncini S, Pecorelli A, Signorini C, Belmonte G, Valacchi G, Rossi M, Hayek J. Ciccoli L, et al. Biochim Biophys Acta. 2012 Apr;1820(4):511-20. doi: 10.1016/j.bbagen.2011.12.002. Epub 2011 Dec 13. Biochim Biophys Acta. 2012. PMID: 22183031 - Subclinical myocardial dysfunction in Rett syndrome.
De Felice C, Maffei S, Signorini C, Leoncini S, Lunghetti S, Valacchi G, D'Esposito M, Filosa S, Della Ragione F, Butera G, Favilli R, Ciccoli L, Hayek J. De Felice C, et al. Eur Heart J Cardiovasc Imaging. 2012 Apr;13(4):339-45. doi: 10.1093/ejechocard/jer256. Epub 2011 Nov 23. Eur Heart J Cardiovasc Imaging. 2012. PMID: 22113206 - Exploring the possible link between MeCP2 and oxidative stress in Rett syndrome.
Filosa S, Pecorelli A, D'Esposito M, Valacchi G, Hajek J. Filosa S, et al. Free Radic Biol Med. 2015 Nov;88(Pt A):81-90. doi: 10.1016/j.freeradbiomed.2015.04.019. Epub 2015 May 8. Free Radic Biol Med. 2015. PMID: 25960047 Review. - F(2)-Dihomo-isoprostanes and brain white matter damage in stage 1 Rett syndrome.
Durand T, De Felice C, Signorini C, Oger C, Bultel-Poncé V, Guy A, Galano JM, Leoncini S, Ciccoli L, Pecorelli A, Valacchi G, Hayek J. Durand T, et al. Biochimie. 2013 Jan;95(1):86-90. doi: 10.1016/j.biochi.2012.09.017. Epub 2012 Sep 23. Biochimie. 2013. PMID: 23009927 Review.
Cited by
- Altered Bone Status in Rett Syndrome.
Pecorelli A, Cordone V, Schiavone ML, Caffarelli C, Cervellati C, Cerbone G, Gonnelli S, Hayek J, Valacchi G. Pecorelli A, et al. Life (Basel). 2021 Jun 3;11(6):521. doi: 10.3390/life11060521. Life (Basel). 2021. PMID: 34205017 Free PMC article. Review. - NaHS Protects against the Impairments Induced by Oxygen-Glucose Deprivation in Different Ages of Primary Hippocampal Neurons.
Yu Q, Wang B, Zhao T, Zhang X, Tao L, Shi J, Sun X, Ding Q. Yu Q, et al. Front Cell Neurosci. 2017 Mar 7;11:67. doi: 10.3389/fncel.2017.00067. eCollection 2017. Front Cell Neurosci. 2017. PMID: 28326019 Free PMC article. - Chondroptosis in alkaptonuric cartilage.
Millucci L, Giorgetti G, Viti C, Ghezzi L, Gambassi S, Braconi D, Marzocchi B, Paffetti A, Lupetti P, Bernardini G, Orlandini M, Santucci A. Millucci L, et al. J Cell Physiol. 2015 May;230(5):1148-57. doi: 10.1002/jcp.24850. J Cell Physiol. 2015. PMID: 25336110 Free PMC article. - Comprehensive Transcriptomic Investigation of Rett Syndrome Reveals Increasing Complexity Trends from Induced Pluripotent Stem Cells to Neurons with Implications for Enriched Pathways.
Odabasi YC, Yanasik S, Saglam-Metiner P, Kaymaz Y, Yesil-Celiktas O. Odabasi YC, et al. ACS Omega. 2023 Nov 8;8(46):44148-44162. doi: 10.1021/acsomega.3c06448. eCollection 2023 Nov 21. ACS Omega. 2023. PMID: 38027357 Free PMC article. - Modelling phenotypes, variants and pathomechanisms of syndromic diseases in different systems.
Gregor A, Zweier C. Gregor A, et al. Med Genet. 2024 Jun 6;36(2):121-131. doi: 10.1515/medgen-2024-2020. eCollection 2024 Jun. Med Genet. 2024. PMID: 38854643 Free PMC article.
References
- Hagberg B, Aicardi J, Dias K, Ramos O. A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: rett’s syndrome: report of 35 cases. Annals of Neurology. 1983;14(4):471–479. - PubMed
- Bienvenu T, Philippe C, De Roux N, et al. The incidence of rett syndrome in France. Pediatric Neurology. 2006;34(5):372–375. - PubMed
- Hagberg B. Clinical manifestations and stages of Rett syndrome. Mental Retardation and Developmental Disabilities Research Reviews. 2002;8(2):61–65. - PubMed
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl—CpG—binding protein 2. Nature Genetics. 1999;23(2):185–188. - PubMed
- Christodoulou J, Grimm A, Maher T, Bennetts B. RettBASE: the IRSA MECP2 variation database-a new mutation database in evolution. Human Mutation. 2003;21(5):466–472. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous