Human ISWI complexes are targeted by SMARCA5 ATPase and SLIDE domains to help resolve lesion-stalled transcription - PubMed (original) (raw)
Human ISWI complexes are targeted by SMARCA5 ATPase and SLIDE domains to help resolve lesion-stalled transcription
Özge Z Aydin et al. Nucleic Acids Res. 2014 Jul.
Abstract
Chromatin compaction of deoxyribonucleic acid (DNA) presents a major challenge to the detection and removal of DNA damage. Helix-distorting DNA lesions that block transcription are specifically repaired by transcription-coupled nucleotide excision repair, which is initiated by binding of the CSB protein to lesion-stalled RNA polymerase II. Using live cell imaging, we identify a novel function for two distinct mammalian ISWI adenosine triphosphate (ATP)-dependent chromatin remodeling complexes in resolving lesion-stalled transcription. Human ISWI isoform SMARCA5/SNF2H and its binding partners ACF1 and WSTF are rapidly recruited to UV-C induced DNA damage to specifically facilitate CSB binding and to promote transcription recovery. SMARCA5 targeting to UV-C damage depends on transcription and histone modifications and requires functional SWI2/SNF2-ATPase and SLIDE domains. After initial recruitment to UV damage, SMARCA5 re-localizes away from the center of DNA damage, requiring its HAND domain. Our studies support a model in which SMARCA5 targeting to DNA damage-stalled transcription sites is controlled by an ATP-hydrolysis-dependent scanning and proofreading mechanism, highlighting how SWI2/SNF2 chromatin remodelers identify and bind nucleosomes containing damaged DNA.
© The Author(s) 2014. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures
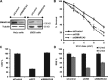
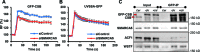
Figure 1.
SMARCA5 functions in transcription-coupled repair. (A) Immunoblots show reduced SMARCA5 expression levels in HeLa cells stably expressing shRNA and U2OS cells treated with siRNA against SMARCA5. Tubulin was used as loading control. (B) SMARCA5 depletion sensitizes cells to UV. Colony survival of HeLa cells stably expressing shRNA against SMARCA5 or CSB following UV irradiation. The percentage of surviving cells is plotted against the applied UV-C dose (J/m2). (C) DNA repair synthesis (UDS) after UV irradiation (16 J/m2), determined by EdU incorporation, as a measure for GG-NER, in wild-type primary fibroblasts (C5RO) (>100 cells for each sample) treated with siRNA. Plotted are, respectively, control (set at 100% UDS), XPC and SMARCA5 siRNAs. (D) Recovery of RNA synthesis (RRS), as a measure for TC-NER, determined by EU incorporation 16 h after UV irradiation (0 and 6 J/m2) in HeLa cells (>100 cells) treated with, respectively, control (set at 100% at 0 J/m2), CSB and SMARCA5 siRNAs. Error bars denote standard error of the mean. The results of all experiments were confirmed at least twice.
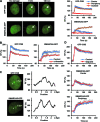
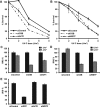
Figure 2.
Transcription-dependent SMARCA5 (re)localization to UV-C damage. (A) Live cell images (left) before, 5 and 140 s after UV-C (266 nm) laser-induced local damage (arrows) of GFP-CSB (expressed in CSB-deficient CS1AN fibroblasts) and SMARCA5-GFP (expressed in U2OS cells). Scale bar is 5 μm. Graphs (right) show the normalized fluorescence intensities (n > 10 cells) that indicate recruitment to the damage center (blue), the damage periphery (orange) and outside the damaged area (red; mean ± standard error of the mean) of GFP-CSB (top) and SMARCA5-GFP. (B) Treatment with α-amanitin impairs the binding to DNA damage sites of GFP-CSB (P < 0.01 compared to control) and SMARCA5-GFP (peripheral recruitment, _P_ = 0.018 compared to control)). (**C**) Line scans of GFP-CSB and SMARCA5-GFP intensity along the indicated line in the image (_n_ = 5 cells). (**D**) GFP-CSB (_P_ = 0.363 compared to control) and SMARCA5-GFP recruitment (center _P_ = 0.682, periphery _P_ = 0.36 compared to control) to DNA damage is unaffected upon PARP inhibition using olaparib (_n_ > 10 cells, mean ± standard error of the mean). RF denotes ‘relative fluorescence’. All results were confirmed using independent, duplicate experiments.
Figure 3.
DNA damage association of SMARCA5 requires histone modifications but not NER. (A) SMARCA5-GFP recruitment is not affected by siRNA-mediated knock-down of CSB in U2OS cells (P = 0.97 compared to control). (B,C) SMARCA5-GFP recruitment to DNA damage, both at the center (left) and at the periphery (right), is impaired after HDAC inhibition by TSA (center P = 0.006, periphery P = 0.040 compared to control) and Na-Bu (center P = 0.006, periphery P = 0.187 compared to control) treatment (B) and by methyltransferase inhibition using Adox [(C); 100-μM center P = 0.064, periphery P = 0.041; 20-μM center P = 0.197, periphery P = 0.076 compared to control]. For each experiment, the mean of n > 10 cells ± standard error of the mean is shown. Graphs depict the normalized fluorescence intensity indicating DNA damage recruitment at the damage center or periphery. Results were confirmed using independent, duplicate experiments. RF denotes ‘relative fluorescence’.
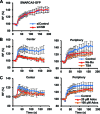
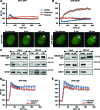
Figure 4.
The ATPase, SLIDE and HAND domains of SMARCA5 regulate its damage recruitment. (A) Schematic representation of SMARCA5 domains (left). The invariant Lysine 211 in the ATPase domain is indicated by a ‘K’. Representative images (right) show the live cell accumulation pattern of wild type (WT), ATPase-dead, HAND, SANT and SLIDE deletions mutants at local UV-C damage in U2OS cells. Scale bar is 5 μm. (B) The SMARCA5 ATPase mutant shows impaired recruitment to DNA damage. Graph of the normalized fluorescence intensity of wild type and ATPase mutant (MT) at the damage center (left) and periphery (right; mean ± standard error of the mean; n > 10 cells). (C) Graphs of the normalized fluorescence intensity of wild type (WT), HAND, SANT and SLIDE deletion mutants (MT) at the damage center (left) and periphery (right; mean ± standard error of the mean; n > 10 cells). Recruitment of the SLIDE domain mutant is impaired, whereas the HAND domain mutant is only recruited to the center of damage. All results were confirmed using independent, duplicate experiments. RF denotes ‘relative fluorescence’.
Figure 5.
SMARCA5 interacts with CSB and regulates its recruitment. (A, B) Graphs of the normalized fluorescence intensity indicating local UV-C-laser-induced DNA damage recruitment of (A) GFP-CSB (P < 0.01 compared to control) and (B) UVSSA-GFP (_P_ = 0.513 compared to control) in cells siRNA depleted for SMARCA5. _n_ > 10 cells, error bars denote standard error of the mean. RF denotes ‘relative fluorescence’. (C) GFP immunoprecipitation of GFP-CSB in MNase-treated nuclear extracts shows that SMARCA5, ACF1 and WSTF co-purify with CSB, both in unchallenged conditions (−UV) and 20 min after UV irradiation (+UV). Ctrl is control. All results were confirmed using independent, duplicate experiments.
Figure 6.
ACF1 and WSTF function in the transcription-coupled response to UV. Depletion of ACF1 and WSTF renders cells hypersensitive to UV and impairs RRS. Colony survival of U2OS cells treated with siRNAs against ACF1 and CSB (A) and HeLa cells stably expressing shRNAs against WSTF and CSB (B). The percentage of surviving cells is plotted against the applied UV-C dose (J/m2). (C,D) Impaired RRS, 16 h after 6 J/m2 UV-C irradiation, in U2OS cells treated with siRNA against ACF1 or WSTF as measured by EU incorporation. (E) siRNA treatment against ACF1 or WSTF in primary C5RO fibroblasts does not affect UDS, as measured by EdU incorporation after 16 J/m2 UV-C irradiation. Error bars denote standard error of the mean. All results were confirmed using independent, duplicate experiments.
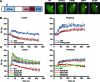
Figure 7.
WSTF and ACF1 are recruited to UV damage to regulate CSB recruitment. ACF1-GFP (A) and GFP-WSTF (B) are recruited to DNA damage induced by UV-C (266 nm) laser. Graphs depict normalized fluorescence intensities indicating DNA damage recruitment in the damage center (blue), the damage periphery (orange) and outside the damaged area (red) (mean ± standard error of the mean; n > 10 cells). Representative images of the accumulation of ACF1-GFP and GFP-WSTF at sites of UV damage are shown below the graphs (scale bar is 5 μm). (C) GFP immunoprecipitation of GFP-tagged SMARCA5 (WT) and ATPase (ATP), HAND and SLIDE domain deletion mutants. CTRL is control. Only deletion of the SLIDE domain impairs the interaction of SMARCA5 with ACF1 and WSTF. (D,E) Graphs of the normalized fluorescence intensity indicating DNA damage recruitment of GFP-CSB in cells in which ACF1 [(D); P = 0.033 compared to control] or WSTF [(E); P = 0.117 compared to control] is depleted by siRNA (mean ± standard error of the mean; n > 10 cells). Results were confirmed using independent, duplicate experiments.
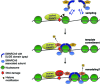
Figure 8.
Model for ISWI recruitment and function in TC-NER. SMARCA5 utilizes ATP hydrolysis and its SLIDE domain, which is necessary for the association with ACF1 and WSTF subunits, to scan for and bind to target nucleosomes in the vicinity of lesion-stalled RNApolII. Its recruitment depends on both active transcription and histone modifications. SMARCA5 may remodel chromatin to facilitate efficient CSB association with stalled transcription sites. See the discussion section for details.
Similar articles
- ISWI chromatin remodeling complexes in the DNA damage response.
Aydin ÖZ, Vermeulen W, Lans H. Aydin ÖZ, et al. Cell Cycle. 2014;13(19):3016-25. doi: 10.4161/15384101.2014.956551. Cell Cycle. 2014. PMID: 25486562 Free PMC article. - Poly(ADP-ribosyl)ation links the chromatin remodeler SMARCA5/SNF2H to RNF168-dependent DNA damage signaling.
Smeenk G, Wiegant WW, Marteijn JA, Luijsterburg MS, Sroczynski N, Costelloe T, Romeijn RJ, Pastink A, Mailand N, Vermeulen W, van Attikum H. Smeenk G, et al. J Cell Sci. 2013 Feb 15;126(Pt 4):889-903. doi: 10.1242/jcs.109413. Epub 2012 Dec 21. J Cell Sci. 2013. PMID: 23264744 - Binding kinetics of human ISWI chromatin-remodelers to DNA repair sites elucidate their target location mechanism.
Erdel F, Rippe K. Erdel F, et al. Nucleus. 2011 Mar-Apr;2(2):105-12. doi: 10.4161/nucl.2.2.15209. Nucleus. 2011. PMID: 21738833 Free PMC article. - ISWI chromatin remodeling: one primary actor or a coordinated effort?
Bartholomew B. Bartholomew B. Curr Opin Struct Biol. 2014 Feb;24:150-5. doi: 10.1016/j.sbi.2014.01.010. Epub 2014 Feb 19. Curr Opin Struct Biol. 2014. PMID: 24561830 Free PMC article. Review. - Structure, function and regulation of CSB: a multi-talented gymnast.
Lake RJ, Fan HY. Lake RJ, et al. Mech Ageing Dev. 2013 May-Jun;134(5-6):202-11. doi: 10.1016/j.mad.2013.02.004. Epub 2013 Feb 16. Mech Ageing Dev. 2013. PMID: 23422418 Free PMC article. Review.
Cited by
- USP3 promotes DNA damage response and chemotherapy resistance through stabilizing and deubiquitinating SMARCA5 in prostate cancer.
Li S, Xiong S, Li Z, Yang L, Yang H, Xiong J, Pan W, Guo J, Xu S, Fu B. Li S, et al. Cell Death Dis. 2024 Nov 5;15(11):790. doi: 10.1038/s41419-024-07117-3. Cell Death Dis. 2024. PMID: 39500888 Free PMC article. - The emerging role of ISWI chromatin remodeling complexes in cancer.
Li Y, Gong H, Wang P, Zhu Y, Peng H, Cui Y, Li H, Liu J, Wang Z. Li Y, et al. J Exp Clin Cancer Res. 2021 Nov 4;40(1):346. doi: 10.1186/s13046-021-02151-x. J Exp Clin Cancer Res. 2021. PMID: 34736517 Free PMC article. Review. - SIRT6 facilitates directional telomere movement upon oxidative damage.
Gao Y, Tan J, Jin J, Ma H, Chen X, Leger B, Xu J, Spagnol ST, Dahl KN, Levine AS, Liu Y, Lan L. Gao Y, et al. Sci Rep. 2018 Mar 29;8(1):5407. doi: 10.1038/s41598-018-23602-0. Sci Rep. 2018. PMID: 29599436 Free PMC article. - SHU00238 Promotes Colorectal Cancer Cell Apoptosis Through miR-4701-3p and miR-4793-3p.
Wang H, Ma Y, Lin Y, Chen R, Xu B, Deng J. Wang H, et al. Front Genet. 2020 Jan 10;10:1320. doi: 10.3389/fgene.2019.01320. eCollection 2019. Front Genet. 2020. PMID: 31998373 Free PMC article. - Facilitation of base excision repair by chromatin remodeling.
Hinz JM, Czaja W. Hinz JM, et al. DNA Repair (Amst). 2015 Dec;36:91-97. doi: 10.1016/j.dnarep.2015.09.011. Epub 2015 Sep 16. DNA Repair (Amst). 2015. PMID: 26422134 Free PMC article. Review.
References
- Hoeijmakers J.H. DNA damage, aging, and cancer. N. Engl. J. Med. 2009;361:1475–1485. - PubMed
- Sugasawa K. Regulation of damage recognition in mammalian global genomic nucleotide excision repair. Mutat. Res. 2010;685:29–37. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous