Islet is a key determinant of ascidian palp morphogenesis - PubMed (original) (raw)
Islet is a key determinant of ascidian palp morphogenesis
Eileen Wagner et al. Development. 2014 Aug.
Abstract
The anterior-most ectoderm of ascidian larvae contains the adhesive papillae, or palps, which play an important role in triggering the metamorphosis of swimming tadpoles. In Ciona intestinalis, the palps consist of three conical protrusions within a field of thickened epithelium that form late in embryogenesis, as tailbuds mature into larvae. The palp protrusions express the LIM-homeodomain transcription factor Islet. Protrusion occurs through differential cell elongation, probably mediated by Islet, as we find that ectopic expression of Islet is sufficient to promote cell lengthening. FGF signaling is required for both Islet expression and palp morphogenesis. Importantly, we show that Islet expression can rescue the palp-deficient phenotype that results from inhibition of FGF signaling. We conclude that Islet is a key regulatory factor governing morphogenesis of the palps. It is conceivable that Islet is also essential for the cellular morphogenesis of placode-derived sensory neurons in vertebrates.
Keywords: Ascidian; Cell shape; Islet; LIM domain; Palps; Placode.
© 2014. Published by The Company of Biologists Ltd.
Figures
Fig. 1.
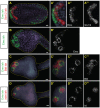
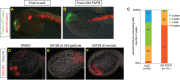
Emx, Btd and Islet mark the presumptive palps. (A) Double ISH of late neurula stage embryo. Emx transcripts detected with fluorescein-labeled probe (green) are expressed in the anterior-most neural plate, as well as the posterior (tail) ectoderm. Six1/2 transcripts detected with DIG-labeled probe (red) are expressed in anterior neural plate posterior to the Emx expression domain. (B) Late tailbud stage embryo hybridized with Emx probe (DIG-labeled). Emx is expressed in three rings that delimit the presumptive palp protrusions, and also weakly in the dorsoanterior ectoderm. (C) Double ISH, late tailbud embryo. Emx transcript is detected with fluorescein-labeled probe (green); two of three presumptive palps are shown. Btd transcripts are detected with DIG-labeled probe (red) and occur with Emx in the rings and also in ectoderm between the rings. (D) Double ISH of late tailbud embryo. Islet probe (DIG-labeled, red) is detected in spots corresponding to the presumptive palp protrusions and is flanked by rings of Emx expression (fluorescein-labeled, green). (A′,B′,C′,D′) Close-up views of corresponding panels, focused on palp region. Nuclei are stained with Hoechst 33342. (A″,A‴,B′,C″,C‴,D″,D‴) In situ signal of respective single probes shown for clarity. Scale bars: 10 μm.
Fig. 2.
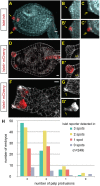
Islet expression correlates with palp protrusion. (A) In situ hybridization reveals Islet transcript in the three presumptive palps at late tailbud stage. Image shown is a confocal _z_-projection of 15 slices. Two of the three presumptive palps, indicated by single and double arrows, are shown in magnified view in panels B-C′. (B-C′) Confocal _z_-projections of two slices focused on an individual palp protrusion. (B,C) Hoechst 33342 counterstaining allows visualization of protruding palp. (B′,C′) Same images as in B and C, but with Islet in situ signal overlaid. (D-E′) Islet>mCherry reporter is expressed in the palps of late tail bud embryo. Image shown is a confocal _z_-projection of four slices focused on one palp. Phalloidin staining reveals cell cortices. Cells expressing Islet reporter are elongated in comparison to neighboring non-expressing cells. (F-G′) Islet>mCherry reporter expression in a mature larva, with phalloidin counterstaining. G shows phalloidin only; arrow indicates digitiform protrusions at tips of palps. G′ shows Islet reporter only, revealing that the digitiform protrusions (arrow) derive from the Islet+ cells. (H) Correlation between protrusion of palps and Islet expression. Late tailbud embryos were electroporated with Islet>mCherry reporter plasmid and stained with phalloidin (as in D). The number of palp protrusions and the number of spots of Islet expression were documented. Data shown are summed from three biological replicates, 249 embryos in total were scored. en, endoderm. Scale bars: 10 μm.
Fig. 3.
Emx and Islet exert differential effects on both Islet expression and palp development. (A-D,F,G) Embryos were electroporated with FoxC>H2B:Cherry and Islet>YFPcaax reporter plasmids and stained with phalloidin. (A) Control embryo expressing FoxC>lacZ shows Islet reporter expression in protrusive palps. (B) Mosaic embryo shows expression of the Islet reporter and protrusion of the palps are repressed in the presence of full-length Emx, whereas protrusion occurs normally on the unelectroporated side. (C) Mosaic embryo expressing FoxC>Emx:WRPW (constitutive repressor) does not express Islet reporter in the palps and does not protrude palps on the electroporated side. (D) Expression of FoxC>Islet leads to a single palp protrusion and ectopic activation of Islet reporter. (E) Quantification of results shown in A-C. To the right (3 palps, 2 palps, etc.) is shown the number of discrete foci detected that expressed the Islet reporter. (F) Mosaic embryo expressing FoxC>Islet DBD:WRPW (constitutive repressor) shows repression of Islet reporter in the palps and loss of protrusion on the perturbed side. (G) Co-expression of FoxC>Emx and FoxC>Islet leads to a single, protrusive palp and ectopic activation of Islet reporter. Scale bars: 10 μm.
Fig. 4.
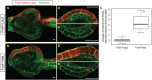
Islet promotes cell elongation. Embryos expressing FoxF>Cherry-caax and FoxF>lacZ (A) or FoxF>Islet (B) were stained with phalloidin and examined by confocal microscopy. A′ and A″ show close-up view of surface ectodermal cells (shown in white brackets) on electroporated and unelectroporated sides, respectively, of the FoxF>lacZ control. Cells on either side are of similar length. B′ and B″ show magnified view of cells on the electroporated and unelectroporated sides of embryos expressing FoxF>Islet. Cells on electroporated side are markedly elongated in comparison to the unperturbed cells. (C) Box plot summary of cell length measurements of 20 embryos, each expressing either FoxF>lacZ or FoxF>Islet. Measurements were performed manually with ImageJ software (all images with measurements are shown in the
supplementary material
). Statistical significance calculated with Wilcoxon two-sample test, _P_=6.8×10−8. Scale bars: 10 μm.
Fig. 5.
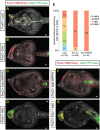
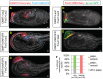
Establishment of Islet pattern requires FGF-MAPK signaling through neurula stage. (A,B) Islet>mCherry reporter was co-expressed with FoxC>YFP-caax and either FoxC>lacZ (A) or FoxC>DN FGFR (B). Embryos were scored for Islet reporter expression at mid-tailbud stage, quantification shown in C. (D-F) Islet ISH of mid-tailbud stage embryos. Embryos were treated with U0126 at mid-gastrula stage (E) or at the early- to mid-neurula stage (F), and compared with control embryos incubated with DMSO (D). Nuclei are stained with Hoechst 33342.
Fig. 6.
Islet expression rescues palp development downstream of perturbed FGF-MAPK signaling. (A-C) Larvae electroporated with DMRT>Cherry-caax and FoxC>H2B:CFP to label anterior neural plate and palp lineage, respectively. (A) Control larva expressing DMRT>lacZ and FoxC>lacZ shows normal palp development. (B) Expression of DMRT>DN FGFR leads to loss of palps, concomitant with ectopic FoxC expression that develops at the expense of the anterior sensory vesicle. (C) Expression of DMRT>DN FGFR and FoxC>Islet leads to formation of one large, protrusive palp. (D,E) Larvae expressing FoxC>H2B:mCherry and palp differentiation marker βγ-crystallin>GFP. Control larva in D shows βγ-crystallin reporter in two of the three palps but not in the intervening palp domain marked by FoxC reporter. (E) The single giant palp in larva expressing DMRT>DN FGFR plus FoxC>Islet shows ectopic expression of βγ-crystallin reporter, concomitant with the FoxC expression domain. (F) Quantification of results shown in D,E. Scale bars: 10 μm.
Fig. 7.
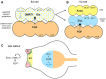
Summary of gene regulatory network underlying palp development. Based on results of current and previous studies (Ikeda et al., 2013; Imai et al., 2006; Wagner and Levine, 2012). Genes in bold black font are expressed in indicated region; gray font indicates genes repressed in those areas; blue font denotes untested regulatory interactions; parentheses indicate genes that were expressed in progenitors of that region but have since been downregulated. (A,B) A subset of the neural progenitors at indicated stages. Dotted vertical line denotes midline. Blastomere names (according to ascidian nomenclature) indicated on right side. Lowercase and uppercase letters (e.g. a7._ and A7._) refer to cells of animal and vegetal hemispheres, respectively. (A) At 64-cell stage, the bipotent palp/CNS progenitors express DMRT1 and Otx in response to FGF signal from vegetal neural precursors. Bipotency requires active repression of ZicL by BZ1/BZ2. (B) At 112-cell stage, the palp and anterior CNS fates have segregated. Anterior CNS fate requires FGF signaling to induce ZicL and restrict FoxC to the palp lineage. (C) In late tailbud embryos, Six3/6 is expressed in anterior brain, downstream of ZicL. (We speculate that Islet and Btd might be actively repressed in the anterior brain by Six3/6. This is because ectopic expression of ZicL in palp progenitors leads to repression of Islet and Btd, and this effect might be mediated by the Six3/6 repressor.) The palps are the anterior-most region of specialized ectoderm (derived from FoxC+ population shown in B) expressing Btd and Emx, as well as the protrusions expressing Islet. Emx might function to limit Islet to discrete foci. Islet is expressed in response to FGF signaling (but see Fig. 5F) and is maintained by positive autoregulation.
Similar articles
- Identification and developmental expression of Ci-isl, a homologue of vertebrate islet genes, in the ascidian Ciona intestinalis.
Giuliano P, Marino R, Pinto MR, De Santis R. Giuliano P, et al. Mech Dev. 1998 Nov;78(1-2):199-202. doi: 10.1016/s0925-4773(98)00143-9. Mech Dev. 1998. PMID: 9858732 - A time delay gene circuit is required for palp formation in the ascidian embryo.
Ikeda T, Matsuoka T, Satou Y. Ikeda T, et al. Development. 2013 Dec;140(23):4703-8. doi: 10.1242/dev.100339. Development. 2013. PMID: 24255097 - Diverse ETS transcription factors mediate FGF signaling in the Ciona anterior neural plate.
Gainous TB, Wagner E, Levine M. Gainous TB, et al. Dev Biol. 2015 Mar 15;399(2):218-25. doi: 10.1016/j.ydbio.2014.12.032. Epub 2015 Jan 7. Dev Biol. 2015. PMID: 25576927 Free PMC article. - Ascidians as excellent chordate models for studying the development of the nervous system during embryogenesis and metamorphosis.
Sasakura Y, Mita K, Ogura Y, Horie T. Sasakura Y, et al. Dev Growth Differ. 2012 Apr;54(3):420-37. doi: 10.1111/j.1440-169X.2012.01343.x. Dev Growth Differ. 2012. PMID: 22524611 Review. - [Recent advances in developmental genomics of the ascidian Ciona intestinalis].
Kawashima T, Shoguchi E, Satou Y, Satoh N. Kawashima T, et al. Tanpakushitsu Kakusan Koso. 2005 Dec;50(16 Suppl):2153-9. Tanpakushitsu Kakusan Koso. 2005. PMID: 16411445 Review. Japanese. No abstract available.
Cited by
- BMP signaling is required to form the anterior neural plate border in ascidian embryos.
Liu B, Ren X, Satou Y. Liu B, et al. Dev Genes Evol. 2023 Jun;233(1):13-23. doi: 10.1007/s00427-023-00702-0. Epub 2023 Apr 20. Dev Genes Evol. 2023. PMID: 37079132 - Shared evolutionary origin of vertebrate neural crest and cranial placodes.
Horie R, Hazbun A, Chen K, Cao C, Levine M, Horie T. Horie R, et al. Nature. 2018 Aug;560(7717):228-232. doi: 10.1038/s41586-018-0385-7. Epub 2018 Aug 1. Nature. 2018. PMID: 30069052 Free PMC article. - Cis-regulatory interfaces reveal the molecular mechanisms underlying the notochord gene regulatory network of Ciona.
Negrón-Piñeiro LJ, Wu Y, Popsuj S, José-Edwards DS, Stolfi A, Di Gregorio A. Negrón-Piñeiro LJ, et al. Nat Commun. 2024 Apr 8;15(1):3025. doi: 10.1038/s41467-024-46850-3. Nat Commun. 2024. PMID: 38589372 Free PMC article. - Stimulatory and inhibitory G-protein signaling relays drive cAMP accumulation for timely metamorphosis in the chordate Ciona.
Hozumi A, Totsuka NM, Onodera A, Wang Y, Hamada M, Shiraishi A, Satake H, Horie T, Hotta K, Sasakura Y. Hozumi A, et al. Elife. 2025 Jun 18;13:RP99825. doi: 10.7554/eLife.99825. Elife. 2025. PMID: 40531176 Free PMC article. - Bioadhesion in ascidians: a developmental and functional genomics perspective.
Pennati R, Rothbächer U. Pennati R, et al. Interface Focus. 2015 Feb 6;5(1):20140061. doi: 10.1098/rsfs.2014.0061. Interface Focus. 2015. PMID: 25657840 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases