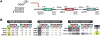Using targeted chromatin regulators to engineer combinatorial and spatial transcriptional regulation - PubMed (original) (raw)
Using targeted chromatin regulators to engineer combinatorial and spatial transcriptional regulation
Albert J Keung et al. Cell. 2014.
Abstract
The transcription of genomic information in eukaryotes is regulated in large part by chromatin. How a diverse array of chromatin regulator (CR) proteins with different functions and genomic localization patterns coordinates chromatin activity to control transcription remains unclear. Here, we take a synthetic biology approach to decipher the complexity of chromatin regulation by studying emergent transcriptional behaviors from engineered combinatorial, spatial, and temporal patterns of individual CRs. We fuse 223 yeast CRs to programmable zinc finger proteins. Site-specific and combinatorial recruitment of CRs to distinct intralocus locations reveals a range of transcriptional logic and behaviors, including synergistic activation, long-range and spatial regulation, and gene expression memory. Comparing these transcriptional behaviors with annotated CR complex and function terms provides design principles for the engineering of transcriptional regulation. This work presents a bottom-up approach to investigating chromatin-mediated transcriptional regulation and introduces chromatin-based components and systems for synthetic biology and cellular engineering.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
Figure 1. A synthetic biology approach to engineering chromatin-based transcriptional regulation
Eukaryotic gene transcription is regulated by diverse chromatin-regulating complexes and networks (top right). The complexes were decomposed into a library of subunit chromatin regulator (CR) proteins (top left). These subunits were fused to engineered zinc finger (ZF) proteins to enable site-specific spatial and combinatorial targeting to designed gene loci (bottom). This modular framework allows the direct functional characterization of individual CRs as transcriptional regulators, and for designing locus architectures that recruit different combinations of CRs to explore and engineer complex spatial and combinatorial transcriptional regulation.
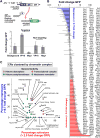
Figure 2. Identifying transcriptional regulators by direct recruitment of a library of 223 CRs
(A, top) 223 CR proteins were fused to an engineered ZF protein (or non-targeting ZF) and placed under the control of an inducible GAL1 promoter. Each fusion protein was individually recruited to operators placed upstream of a minimal CYC1 promoter driving the expression of GFP. NLS, nuclear localization signal. (A, bottom) Fold change in GFP expression induced by VP16 activation and Mig1 repression domains fused to targeting or non-targeting ZF proteins. (B) Fold change in GFP expression for the library of 223 ZF-CR fusions (normalized to uninduced levels). Repressors (blue bars) were classified as having < 0.7 fold change, while activators (red bars) have > 2 fold change. (C) CRs grouped by complex and plotted according to the percentage of activators and repressors in each complex. Dot colors correspond to the general activities of each complex. Error bars are standard deviations of three isogenic strains. See also Figures S1 and S2.
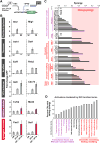
Figure 3. Combinatorial recruitment reveals distinct classes of regulators for engineering transcriptional logic
(A) An engineered two-input system enabling the co-recruitment of CRs and VP16 transactivating domain (ZF 43-8, grey; ZF 97-4, blue) (Khalil et al., 2012). (B) Representative transcriptional logic outputs of the two-input system divide CRs into six distinct classes (top to bottom): VP16-independent dominant repressors, repressors, CRs with no effect, VP16 enhancers, additive activators (purple), and synergistic activators (red). (C) Activating CRs clustered by complex and plotted by level of transcriptional synergy. Transcription/preinitiation complex regulators generated weak synergy, while chromatin assembly/remodeling, chromatin-modifying, and transcription-elongating regulators generated strong synergy. Synergy is the “cooperation” of factors to produce a total output, and here is defined as the fraction of total output not accounted for by summing the outputs from the individual components. Synergy = [(A – 1) – (B – 1) – (C – 1)]/(A – 1) where A = CR and VP16, B = CR only, and C = VP16 only. (D) Activators clustered by gene ontology function terms and plotted as percentage of CRs in each term group with “strong synergy” (greater than the average synergy of 0.2). Error bars are standard deviations of three isogenic strains. See also S3.
Figure 4. Engineering spatial regulation by targeting CRs upstream and downstream of a gene
A gene locus was engineered to recruit 223 CR fusions to operators either upstream or downstream (downstream of a CYC1 terminator) of a reporter gene. CRs were grouped according to their upstream- and downstream-targeted regulatory profiles. Gene ontology function terms unique to each group are listed along with the number of CRs in the group associated with each term. See also Figure S4.
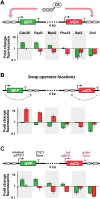
Figure 5. Simultaneous and distinct regulation of two genes by individual CRs
(A) Schematic of the engineered, dual-gene reporter locus (CYC1 promoters and terminators used throughout) (top). Fold change in GFP (green bars) and mCherry (red bars) expression for six targeted CR fusions (bottom). (B) Swapping operator locations results in inversion of transcriptional outputs. (C) Schematic of the same locus architecture as in (A) but containing two different promoters and terminators (BIO2 promoter and ADH1 terminator in purple). Error bars are standard deviations of three isogenic strains. See also Figure S5.
Figure 6. Long-range and multi-gene regulation by targeted CRs
(A) Schematic of the engineered, multi-gene reporter locus. The 27 strongest repressors and 48 strongest activators identified from the full ZF-CR library as well as all CRs with histone-modifying catalytic domains were targeted upstream of the first gene. (B) Heat map of the fold change in fluorescence for GFP, mCherry, and BFP, revealing classes of CRs that regulate only the proximal gene (left and middle) or that repress all three genes in the locus (right). See also Figure S6.
Figure 7. Epigenetic repression and insulation
(A) Time courses of induction/wash-out experiments for three CRs. CR fusions were expressed at t = 0 hr by the addition of the small molecule ATc, which was subsequently washed out at t = 12 hr (grey bars). Med16 and Isw2 show reversible activation and repression of GFP, respectively. Sir2 maintains full repressive memory of the proximal gene and partial repressive memory of the downstream gene. (B) Nucleosome-disfavoring sequences inserted between the GFP and mCherry genes as putative barrier or insulator elements. (C) Fold change in fluorescence for GFP, mCherry, and BFP induced by targeting (top) or non-targeting (bottom) multi-gene repressors (Sir2, Rph1, Sum1 fusions). The (CCGNN)32 sequence robustly insulates only the middle gene (mCherry) from repression by the CRs. (D) Schematic of the multi-gene regulatory circuit. Error bars are standard deviations of three isogenic strains. See also Figure S7.
Comment in
- Gene regulation: a chromatin-based recruitment drive.
Burgess DJ. Burgess DJ. Nat Rev Genet. 2014 Aug;15(8):513. doi: 10.1038/nrg3779. Epub 2014 Jul 15. Nat Rev Genet. 2014. PMID: 25022905 No abstract available.
Similar articles
- A high-throughput synthetic biology approach for studying combinatorial chromatin-based transcriptional regulation.
Alcantar MA, English MA, Valeri JA, Collins JJ. Alcantar MA, et al. Mol Cell. 2024 Jun 20;84(12):2382-2396.e9. doi: 10.1016/j.molcel.2024.05.025. Mol Cell. 2024. PMID: 38906116 - Transcriptional networks: reverse-engineering gene regulation on a global scale.
Chua G, Robinson MD, Morris Q, Hughes TR. Chua G, et al. Curr Opin Microbiol. 2004 Dec;7(6):638-46. doi: 10.1016/j.mib.2004.10.009. Curr Opin Microbiol. 2004. PMID: 15556037 Review. - Transcriptional regulatory network shapes the genome structure of Saccharomyces cerevisiae.
Li S, Heermann DW. Li S, et al. Nucleus. 2013 May-Jun;4(3):216-28. doi: 10.4161/nucl.24875. Epub 2013 May 1. Nucleus. 2013. PMID: 23674068 Free PMC article. - Comprehensive analysis of combinatorial regulation using the transcriptional regulatory network of yeast.
Balaji S, Babu MM, Iyer LM, Luscombe NM, Aravind L. Balaji S, et al. J Mol Biol. 2006 Jun 30;360(1):213-27. doi: 10.1016/j.jmb.2006.04.029. Epub 2006 May 3. J Mol Biol. 2006. PMID: 16762362 - The Yap family and its role in stress response.
Rodrigues-Pousada C, Menezes RA, Pimentel C. Rodrigues-Pousada C, et al. Yeast. 2010 May;27(5):245-58. doi: 10.1002/yea.1752. Yeast. 2010. PMID: 20148391 Review.
Cited by
- Nucleosome distortion as a possible mechanism of transcription activation domain function.
Erkina TY, Erkine AM. Erkina TY, et al. Epigenetics Chromatin. 2016 Sep 20;9:40. doi: 10.1186/s13072-016-0092-2. eCollection 2016. Epigenetics Chromatin. 2016. PMID: 27679670 Free PMC article. Review. - Gene networks that compensate for crosstalk with crosstalk.
Müller IE, Rubens JR, Jun T, Graham D, Xavier R, Lu TK. Müller IE, et al. Nat Commun. 2019 Sep 6;10(1):4028. doi: 10.1038/s41467-019-12021-y. Nat Commun. 2019. PMID: 31492904 Free PMC article. - The INO80 Complex Requires the Arp5-Ies6 Subcomplex for Chromatin Remodeling and Metabolic Regulation.
Yao W, King DA, Beckwith SL, Gowans GJ, Yen K, Zhou C, Morrison AJ. Yao W, et al. Mol Cell Biol. 2016 Jan 11;36(6):979-91. doi: 10.1128/MCB.00801-15. Mol Cell Biol. 2016. PMID: 26755556 Free PMC article. - Utilization of CRISPR-Cas genome editing technology in filamentous fungi: function and advancement potentiality.
Shen Q, Ruan H, Zhang H, Wu T, Zhu K, Han W, Dong R, Ming T, Qi H, Zhang Y. Shen Q, et al. Front Microbiol. 2024 Mar 28;15:1375120. doi: 10.3389/fmicb.2024.1375120. eCollection 2024. Front Microbiol. 2024. PMID: 38605715 Free PMC article. Review. - Synthetic biology devices for in vitro and in vivo diagnostics.
Slomovic S, Pardee K, Collins JJ. Slomovic S, et al. Proc Natl Acad Sci U S A. 2015 Nov 24;112(47):14429-35. doi: 10.1073/pnas.1508521112. Proc Natl Acad Sci U S A. 2015. PMID: 26598662 Free PMC article. Review.
References
- Atkinson MR, Savageau MA, Myers JT, Ninfa AJ. Development of genetic circuitry exhibiting toggle switch or oscillatory behavior in Escherichia coli. Cell. 2003;113:597–607. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases