Stool microbiota and vaccine responses of infants - PubMed (original) (raw)
Observational Study
. 2014 Aug;134(2):e362-72.
doi: 10.1542/peds.2013-3937. Epub 2014 Jul 7.
Affiliations
- PMID: 25002669
- PMCID: PMC4187229
- DOI: 10.1542/peds.2013-3937
Observational Study
Stool microbiota and vaccine responses of infants
M Nazmul Huda et al. Pediatrics. 2014 Aug.
Abstract
Objective: Oral vaccine efficacy is low in less-developed countries, perhaps due to intestinal dysbiosis. This study determined if stool microbiota composition predicted infant oral and parenteral vaccine responses.
Methods: The stool microbiota of 48 Bangladeshi infants was characterized at 6, 11, and 15 weeks of age by amplification and sequencing of the 16S ribosomal RNA gene V4 region and by Bifidobacterium-specific, quantitative polymerase chain reaction. Responses to oral polio virus (OPV), bacille Calmette-Guérin (BCG), tetanus toxoid (TT), and hepatitis B virus vaccines were measured at 15 weeks by using vaccine-specific T-cell proliferation for all vaccines, the delayed-type hypersensitivity skin-test response for BCG, and immunoglobulin G responses using the antibody in lymphocyte supernatant method for OPV, TT, and hepatitis B virus. Thymic index (TI) was measured by ultrasound.
Results: Actinobacteria (predominantly Bifidobacterium longum subspecies infantis) dominated the stool microbiota, with Proteobacteria and Bacteroidetes increasing by 15 weeks. Actinobacteria abundance was positively associated with T-cell responses to BCG, OPV, and TT; with the delayed-type hypersensitivity response; with immunoglobulin G responses; and with TI. B longum subspecies infantis correlated positively with TI and several vaccine responses. Bacterial diversity and abundance of Enterobacteriales, Pseudomonadales, and Clostridiales were associated with neutrophilia and lower vaccine responses.
Conclusions: Bifidobacterium predominance may enhance thymic development and responses to both oral and parenteral vaccines early in infancy, whereas deviation from this pattern, resulting in greater bacterial diversity, may cause systemic inflammation (neutrophilia) and lower vaccine responses. Vaccine responsiveness may be improved by promoting intestinal bifidobacteria and minimizing dysbiosis early in infancy.
Keywords: Actinobacteria; Bangladesh; Bifidobacterium; Proteobacteria; T lymphocyte; antibody; hepatitis; intestinal; microbiota; polio; tetanus; tuberculosis; vaccine.
Copyright © 2014 by the American Academy of Pediatrics.
Figures
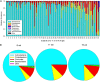
FIGURE 1
A, Relative abundance of microbiota by 16S rRNA gene sequence analysis in each of the 48 subjects (numbered from highest to lowest abundance of Actinobacteria at 6 weeks) at all time points. For each subject, bars are ordered by age (6, 11, and 15 weeks) from left to right. B, Mean relative abundance at 6, 11, and 15 weeks of age.
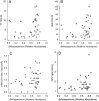
FIGURE 2
Positive association of vaccine responses with stool Actinobacteria at 15 weeks of age. The relative abundance of stool microbiota was determined by sequence analysis of the 16S rRNA gene. Vaccine responses were characterized by the CD4 T-cell SI in response to vaccine antigens for OPV vaccine (A), BCG vaccine using PPD antigen (B), TT vaccine (C), HBV vaccine (D), and SEB (E), the positive control for T-cell stimulation. Box plots (A–D) show the median; 10th, 25th, 75th, and 90th percentiles; and individual outliers. P values (Wilcoxon rank-sum test) for comparisons between groups were as follows: A, .046; B, .036; C, .019; and D, 0.78. E, The scatterplot association was determined by Spearman correlation (R): R = 0.331, P = .034.
FIGURE 3
Positive association of vaccine responses with stool Bifidobacterium, B longum, B longum subspecies infantis, and B longum subspecies longum at 15 weeks of age. Infant vaccine responses were characterized as being above (“high”) or below (“low”) the median vaccine responses for the CD4 SI for OPV (A), the CD8 SI for OPV (B), the CD4 SI for PPD as an index of response to the BCG vaccine (C), and for the positive control for polyclonal T-cell proliferation, the CD4 SI for SEB (D). Differences between those above and below the median were identified by the Wilcoxon rank-sum test as indicated by the superscript letters: a_P_ < .10, b_P_ < .05, c_P_ < .01. The Bifidobacterium genus was identified by PCR, B longum species by T-RFLP, and B longum subspecies infantis and B longum subspecies longum as described in Methods.
FIGURE 4
Positive association of vaccine responses with relative abundance of stool Bifidobacterium determined by sequence analysis of the 16S rRNA gene. Vaccine responses were characterized by CD4 stimulation index response to TT (A), CD4 stimulation index in response to the polyclonal T-cell mitogen SEB (B), the DTH skin-test response to PPD antigen as an index of the response to BCG immunization (C), and the IgG response to the OPV vaccine (D). Spearman correlation coefficients (R) and P values were as follows: A, R = 0.389, P = .013; B, R = 0.315, P = .045; C, R = 0.337, P = .020; and D, R = 0.301, P = .047.
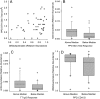
FIGURE 5
Positive association of vaccine responses with stool Bifidobacteriales, Actinomycetales, and Coriobacteriales (from the class Actinobacteria) at 15 weeks of age. The relative abundance of stool microbiota at the order level in the class Actinobacteria was determined by sequence analysis of the 16S rRNA gene at 15 weeks of age. A and B, The response to BCG vaccination was determined by using the DTH skin-test response to PPD antigen. C, The response to TT vaccination was determined by using the antibody in lymphocyte supernatant assay to measure TT-specific IgG levels (TT IgG). D, The CD4 T-cell SI in response to PPD antigen. A, The scatterplot association was determined by Spearman correlation (R): R = 0.333, P = .025. Box plots (B, C, and D) show the median; 10th, 25th, 75th, and 90th percentiles; and individual outliers. P values (Wilcoxon rank-sum test) for comparisons between groups were as follows: B, .018; C, .041; and D, .034.
FIGURE 6
Negative association of stool Pseudomonadales (A), Enterobacteriales (B), and Shannon Diversity Index (measured at the phylum level) (C) with CD4 T-cell SI response to TT vaccine at 15 weeks of age. The relative abundance of stool microbiota was determined by sequence analysis of the 16S rRNA gene. Box plots show the median; 10th, 25th, 75th, and 90th percentiles; and individual outliers. P values (Wilcoxon rank-sum test) for comparisons between groups were as follows: A, 0.022; B, 0.047. C, The Spearman correlation coefficient was R = −0.490 with P = .0013.
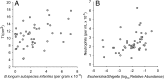
FIGURE 7
Correlation of Bifidobacterium longum subspecies infantis in stool determined by qPCR with TI (A) and relative abundance of Escherichia/Shigella genera in stool determined by sequence analysis of the 16S rRNA gene with blood neutrophil levels (B). Spearman correlation coefficients (R) and P values were as follows: A, R = 0.389, P = .0083; B, R = 0.431, P = .0028.
Similar articles
- Bifidobacterium Abundance in Early Infancy and Vaccine Response at 2 Years of Age.
Huda MN, Ahmad SM, Alam MJ, Khanam A, Kalanetra KM, Taft DH, Raqib R, Underwood MA, Mills DA, Stephensen CB. Huda MN, et al. Pediatrics. 2019 Feb;143(2):e20181489. doi: 10.1542/peds.2018-1489. Pediatrics. 2019. PMID: 30674610 Free PMC article. Clinical Trial. - Influence of Mycobacterium bovis bacillus Calmette-Guérin on antibody and cytokine responses to human neonatal vaccination.
Ota MO, Vekemans J, Schlegel-Haueter SE, Fielding K, Sanneh M, Kidd M, Newport MJ, Aaby P, Whittle H, Lambert PH, McAdam KP, Siegrist CA, Marchant A. Ota MO, et al. J Immunol. 2002 Jan 15;168(2):919-25. doi: 10.4049/jimmunol.168.2.919. J Immunol. 2002. PMID: 11777990 Clinical Trial. - Infant cortisol stress-response is associated with thymic function and vaccine response.
Huda MN, Ahmad SM, Alam MJ, Khanam A, Afsar MNA, Wagatsuma Y, Raqib R, Stephensen CB, Laugero KD. Huda MN, et al. Stress. 2019 Jan;22(1):36-43. doi: 10.1080/10253890.2018.1484445. Epub 2018 Jun 22. Stress. 2019. PMID: 29932814 Free PMC article. Clinical Trial. - The influence of the intestinal microbiome on vaccine responses.
Zimmermann P, Curtis N. Zimmermann P, et al. Vaccine. 2018 Jul 16;36(30):4433-4439. doi: 10.1016/j.vaccine.2018.04.066. Epub 2018 Jun 13. Vaccine. 2018. PMID: 29909134 Review.
Cited by
- Should infants cry over spilled milk? Fecal glycomics as an indicator of a healthy infant gut microbiome.
Frese SA, Mills DA. Frese SA, et al. J Pediatr Gastroenterol Nutr. 2015 Jun;60(6):695. doi: 10.1097/MPG.0000000000000798. J Pediatr Gastroenterol Nutr. 2015. PMID: 26000886 Free PMC article. No abstract available. - Probiotics and probiotic-based vaccines: A novel approach for improving vaccine efficacy.
Kazemifard N, Dehkohneh A, Baradaran Ghavami S. Kazemifard N, et al. Front Med (Lausanne). 2022 Oct 13;9:940454. doi: 10.3389/fmed.2022.940454. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36313997 Free PMC article. Review. - Nuxcell Neo® improves vaccine efficacy in antibody response.
Jesus GFA, Galvani NC, Abel JDS, Scussel R, Fagundes MĹ, Córneo EDS, Rossetto M, Sargiani D, de Ávila RAM, Michels M. Jesus GFA, et al. Front Vet Sci. 2024 Feb 13;11:1248811. doi: 10.3389/fvets.2024.1248811. eCollection 2024. Front Vet Sci. 2024. PMID: 38414656 Free PMC article. - The Gut Microbiome of Children during the COVID-19 Pandemic.
Bacorn M, Romero-Soto HN, Levy S, Chen Q, Hourigan SK. Bacorn M, et al. Microorganisms. 2022 Dec 13;10(12):2460. doi: 10.3390/microorganisms10122460. Microorganisms. 2022. PMID: 36557713 Free PMC article. Review.
References
- Matamoros S, Gras-Leguen C, Le Vacon F, Potel G, de La Cochetiere MF. Development of intestinal microbiota in infants and its impact on health. Trends Microbiol. 2013;21(4):167–173 - PubMed
- Bisgaard H, Li N, Bonnelykke K, et al. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J Allergy Clin Immunol. 2011;128(3):646–652, e641–e645 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical