Methaneseleninic acid and γ-Tocopherol combination inhibits prostate tumor growth in Vivo in a xenograft mouse model - PubMed (original) (raw)
Methaneseleninic acid and γ-Tocopherol combination inhibits prostate tumor growth in Vivo in a xenograft mouse model
Chandra K Singh et al. Oncotarget. 2014.
Abstract
Studies have shown that vitamin E and selenium possess antiproliferative effects against prostate cancer (PCa). However, results from the Selenium and Vitamin E Cancer Prevention Trial (SELECT) suggest that vitamin E (α-tocopheryl acetate; 400 mg) and/or selenium (L-selenomethionine; 200 μg) were ineffective against PCa in humans. It is arguable that the selected dose/formulation of vitamin E/selenium were not optimal in SELECT. Thus, additional studies are needed to define the appropriate formulations/dose regimens of these agents. Here, we investigated the effect of methaneseleninic acid (MSA; 41 µg/kg) and/or γ-tocopherol (γT; 20.8 mg/kg or 41.7 mg/kg) in Nu/J mice implanted with 22Rν1 tumors. MSA (41 µg/kg) and γT (20.8 mg/kg) combination was most consistent in imparting anti-proliferative response; resulting in a significant decrease in i) tumor volume/weight, ii) serum PSA, and iii) Ki-67 immunostaining. Further, we observed i) an upregulation of pro-apoptosis Bax and a down-regulation of the pro-survival Bcl2, and ii) an increase in pro-apoptosis Bad. Furthermore, the combination resulted in a modulation of apolipoprotein E, selenoprotein P and Nrf2 in a fashion that favors antiproliferative responses. Overall, our study suggested that a combination of MSA and γT, at lower dose regimen, could be useful in PCa management.
Figures
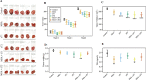
Figure 1. Effects of oral MSA and/or γT on prostate tumor growth in athymic nude mice
Mice were subcutaneously implanted with 22Rν1 cells, and after 12 days MSA and/or γT were administered as described in ‘Materials and Methods’. Effects of MSA and/or γT were assessed for tumor size, tumor volume, tumor wet weight and serum PSA levels. Following sacrifice, tumors were resected. Representative images of each treatment group are shown (A). Tumor volume was measured at the start of the experiment and subsequently at 7th and 14th days after starting treatment (B). At the end of the experiment, tumor volume was determined (C); Average wet weights of resected tumors were taken (D); and serum PSA levels were measured (E). All are represented as mean value ± 2 standard errors of mean (*P<0.05, **P<0.01 and ***P<0.001).
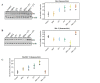
Figure 2. Effects of MSA and/or γT on tumor cell proliferation
At the termination of xenograft experiment, tumors were excised and processed for Ki-67 immunostaining as detailed in ‘Materials and Methods’. Immunohistochemical analysis was performed in tumor tissue of control and treatment groups under bright field microscope. Representative images (400x magnification) of each treatment group are shown.
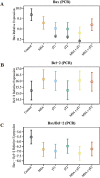
Figure 3. Effects of MSA and γT on Bax and Bcl2 proteins
At the termination of the experiment, the effects of the treatments on Bax and Bcl2 proteins were determined using immunoblot analysis. 40 μg protein was separated on SDS-PAGE and immunobloted for Bax (A) and Bcl2 (B) as detailed in ‘Materials and Methods’. The blots were reprobed for β-actin for loading control. Representative blots are shown and the data (relative density normalized to β-actin) are expressed as mean ± 2 standard error of three replicates (representing 6 mice). Ratio of Bax/Bcl2 was also calculated and plotted (C) (*P<0.05).
Figure 4. Effects of MSA and/or γT on Bax and Bcl2 mRNA
At the termination of xenograft experiment, effects of treatments on Bax and Bcl2 transcription were assessed by qRT-PCR analyses. RNA from tumor tissue samples was isolated and cDNA was made. qRT-PCR was run for Bax and Bcl2 as detailed in ‘Materials and Methods’. GAPDH was used as endogenous control. The qRT-PCR data are represented as relative mRNA levels for Bax (A) and Bcl2 (B). Ratio of Bax/Bcl2 mRNA was calculated and plotted (C). The data represented are mean ± 2 standard error of three replicates (representing 6 mice) (*P<0.05).
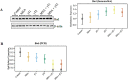
Figure 5. Effects of MSA and/or γT on Bad protein and mRNA
The effects of MSA and γT on pro-apoptosis Bad was analyzed at protein as well as mRNA levels. For immunoblot analysis, 40 μg protein was separated on SDS-PAGE and immunobloted as detailed in ‘Materials and Methods’. The blots were reprobed for β-actin for loading control. Relative protein expression of Bad was calculated and plotted (A). Relative mRNA levels of Bad were assessed using qRT-PCR assay as detailed in ‘Materials and Methods’ (B). The data represented are mean ± 2 standard error of three replicates (representing 6 mice) (*P<0.05).
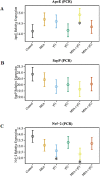
Figure 6. Effects of MSA and/or γT on oxidative stress markers
To assess the effects of MSA and/or γT on modulation of oxidative stress, ApoE, SepP and Nrf2 mRNA level were analyzed. RNA isolation from tumor tissue followed by cDNA synthesis and then qRT-PCR was performed as detailed in ‘Materials and Methods’. The qRT-PCR data are represented as relative quantity (normalized to GAPDH) for ApoE (A), SepP (B) and Nrf2 (C) transcriptional levels. Representative data are expressed as mean ± 2 standard error of three replicates (representing 6 mice) (*P<0.05).
Similar articles
- Combination of vitamin E and selenium causes an induction of apoptosis of human prostate cancer cells by enhancing Bax/Bcl-2 ratio.
Reagan-Shaw S, Nihal M, Ahsan H, Mukhtar H, Ahmad N. Reagan-Shaw S, et al. Prostate. 2008 Nov 1;68(15):1624-34. doi: 10.1002/pros.20824. Prostate. 2008. PMID: 18668529 Free PMC article. - Methylseleninic acid enhances the effect of etoposide to inhibit prostate cancer growth in vivo.
Gonzalez-Moreno O, Segura V, Serrano D, Nguewa P, de las Rivas J, Calvo A. Gonzalez-Moreno O, et al. Int J Cancer. 2007 Sep 15;121(6):1197-204. doi: 10.1002/ijc.22764. Int J Cancer. 2007. PMID: 17520673 - Selenium, but not lycopene or vitamin E, decreases growth of transplantable dunning R3327-H rat prostate tumors.
Lindshield BL, Ford NA, Canene-Adams K, Diamond AM, Wallig MA, Erdman JW Jr. Lindshield BL, et al. PLoS One. 2010 Apr 29;5(4):e10423. doi: 10.1371/journal.pone.0010423. PLoS One. 2010. PMID: 20454690 Free PMC article. - A nutrient approach to prostate cancer prevention: The Selenium and Vitamin E Cancer Prevention Trial (SELECT).
Dunn BK, Richmond ES, Minasian LM, Ryan AM, Ford LG. Dunn BK, et al. Nutr Cancer. 2010;62(7):896-918. doi: 10.1080/01635581.2010.509833. Nutr Cancer. 2010. PMID: 20924966 Review.
Cited by
- Modulation of Metabolic Detoxification Pathways Using Foods and Food-Derived Components: A Scientific Review with Clinical Application.
Hodges RE, Minich DM. Hodges RE, et al. J Nutr Metab. 2015;2015:760689. doi: 10.1155/2015/760689. Epub 2015 Jun 16. J Nutr Metab. 2015. PMID: 26167297 Free PMC article. Review. - Potent inhibitory effect of δ-tocopherol on prostate cancer cells cultured in vitro and grown as xenograft tumors in vivo.
Huang H, He Y, Cui XX, Goodin S, Wang H, Du ZY, Li D, Zhang K, Tony Kong AN, DiPaola RS, Yang CS, Conney AH, Zheng X. Huang H, et al. J Agric Food Chem. 2014 Nov 5;62(44):10752-8. doi: 10.1021/jf504058f. Epub 2014 Oct 27. J Agric Food Chem. 2014. PMID: 25322450 Free PMC article. - Role of Natural and Synthetic Compounds in Modulating NRF2/KEAP1 Signaling Pathway in Prostate Cancer.
Tossetta G, Fantone S, Marzioni D, Mazzucchelli R. Tossetta G, et al. Cancers (Basel). 2023 Jun 2;15(11):3037. doi: 10.3390/cancers15113037. Cancers (Basel). 2023. PMID: 37296999 Free PMC article. Review. - Methylseleninic acid activates Keap1/Nrf2 pathway via up-regulating miR-200a in human oesophageal squamous cell carcinoma cells.
Liu M, Hu C, Xu Q, Chen L, Ma K, Xu N, Zhu H. Liu M, et al. Biosci Rep. 2015 Sep 4;35(5):e00256. doi: 10.1042/BSR20150092. Biosci Rep. 2015. PMID: 26341629 Free PMC article. - Gamma-tocopherol, a major form of vitamin E in diets: Insights into antioxidant and anti-inflammatory effects, mechanisms, and roles in disease management.
Jiang Q, Im S, Wagner JG, Hernandez ML, Peden DB. Jiang Q, et al. Free Radic Biol Med. 2022 Jan;178:347-359. doi: 10.1016/j.freeradbiomed.2021.12.012. Epub 2021 Dec 9. Free Radic Biol Med. 2022. PMID: 34896589 Free PMC article.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. - PubMed
- Clark LC, Dalkin B, Krongrad A, Combs GF, Jr, Turnbull BW, Slate EH, Witherington R, Herlong JH, Janosko E, Carpenter D, Borosso C, Falk S, Rounder J. Decreased incidence of prostate cancer with selenium supplementation: results of a double-blind cancer prevention trial. Br J Urol. 1998;81(5):730–734. - PubMed
- The effect of vitamin E. beta carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N Eng J Med. 1994;330(15):1029–1035. - PubMed
- Heinonen OP, Albanes D, Virtamo J, Taylor PR, Huttunen JK, Hartman AM, Haapakoski J, Malila N, Rautalahti M, Ripatti S, Maenpaa H, Teerenhovi L, Koss L, Virolainen M, Edwards BK. Prostate cancer and supplementation with alpha-tocopherol and beta-carotene: incidence and mortality in a controlled trial. J Natl Cancer Inst. 1998;90(6):440–446. - PubMed
- Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA, Parsons JK, Bearden JD, 3rd, Crawford ED, Goodman GE, Claudio J, Winquist E, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301(1):39–51. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 CA176867/CA/NCI NIH HHS/United States
- R21 CA149560/CA/NCI NIH HHS/United States
- R21CA176867/CA/NCI NIH HHS/United States
- R21CA149560/CA/NCI NIH HHS/United States
- R01 CA114060/CA/NCI NIH HHS/United States
- R01CA114060/CA/NCI NIH HHS/United States
- P30 CA014520/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous