Dual mechanisms by which miR-125b represses IRF4 to induce myeloid and B-cell leukemias - PubMed (original) (raw)
. 2014 Aug 28;124(9):1502-12.
doi: 10.1182/blood-2014-02-553842. Epub 2014 Jul 8.
Reeshelle Sookram 1, Aadel A Chaudhuri 2, Aarathi Minisandram 1, David Cheng 1, Catherine Xie 1, Ee Lyn Lim 1, Yvette Garcia Flores 1, Shuai Jiang 1, Jocelyn Tammy Kim 1, Christopher Keown 3, Parameswaran Ramakrishnan 4, David Baltimore 1
Affiliations
- PMID: 25006123
- PMCID: PMC4148772
- DOI: 10.1182/blood-2014-02-553842
Dual mechanisms by which miR-125b represses IRF4 to induce myeloid and B-cell leukemias
Alex Yick-Lun So et al. Blood. 2014.
Abstract
The oncomir microRNA-125b (miR-125b) is upregulated in a variety of human neoplastic blood disorders and constitutive upregulation of miR-125b in mice can promote myeloid and B-cell leukemia. We found that miR-125b promotes myeloid and B-cell neoplasm by inducing tumorigenesis in hematopoietic progenitor cells. Our study demonstrates that miR-125b induces myeloid leukemia by enhancing myeloid progenitor output from stem cells as well as inducing immortality, self-renewal, and tumorigenesis in myeloid progenitors. Through functional and genetic analyses, we demonstrated that miR-125b induces myeloid and B-cell leukemia by inhibiting interferon regulatory factor 4 (IRF4) but through distinct mechanisms; it induces myeloid leukemia through repressing IRF4 at the messenger RNA (mRNA) level without altering the genomic DNA and induces B-cell leukemia via genetic deletion of the gene encoding IRF4.
© 2014 by The American Society of Hematology.
Figures
Figure 1
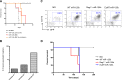
Features of miR-125b-induced myeloid cancer cells. (A) Leukemic cells from MG-125b are serially transplantable. Cells from MG and MG-125b mice were harvested 3 to 6 months after bone marrow reconstitution. 200K GFP+ splenic cells (black dotted line) or 400 to 1000K GFP+ BMCs (red line) from MG-125b mice were transferred into secondary sublethally irradiated C57bl/6 recipients. Two million GFP+ BMCs from the secondary mice were injected into tertiary nonirradiated C57bl/6 recipients (orange line indicated as “no irradiation”). Death point of the recipients (at least 4 mice per group) were recorded when found dead or moribund. (B) Myeloid cancer cells induced by miR-125b overexpression were harvested and subjected to exome deep-sequencing analysis. Every exon was sequenced an average of 50 times. The sequencing data sets were filtered on a 0.1% false-positive discovery rate and cancer-associated mutations were obtained by excluding those that were identified in normal noncancerous mice. Plot shows the number of cancer-associated exonic mutations identified in each of the 3 independent experiments. (C) Donor BMCs from C57bl/6, Rag1−/−, and EμMT (B6.129S2-Ighmtm1Cgn/J) mice were transduced with MG-125b retroviruses, which encode for miR-125b and GFP expression. As control, donor BMCs from C57bl/6 animals were transduced with empty MG retroviruses, which encode for GFP. BMCs were transplanted into lethally irradiated C57bl/6 recipients, and the blood of the mice was subjected to flow cytometric analyses 3 months after reconstitution. (D) The survival curve of the mice described in panel C is shown (3 mice per group). Mice were considered dead when they became moribund or found dead.
Figure 2
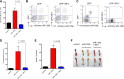
Overexpressing miR-125b in BMCs induces myeloproliferative disorder in vitro. (A) HSPC-enriched BMCs, which were obtained by injecting C57bl/6 mice with 5-fluorouracil, were transduced with MG control or MG-125b retroviruses. Equal numbers of BMCs were cultured in 50 ng/mL SCF, 50 ng/mL IL-6, and 25 ng/mL IL-3. After 5 days, the total number of myeloid cells (CD11b+) cells was determined by flow cytometry. (B) MG control or miR-125b–overexpressing HSPC-enriched cells were expanded at same starting density in 50 ng/mL SCF. Cells were counted by flow cytometry. (C) Equal numbers of HSPC-enriched cells transduced with MG or miR-125b overexpression cassette were expanded in 50 ng/mL SCF. The density of Lin−Sca1−cKit+ cells after 4 days was determined by flow cytometry. (D) Equal numbers of MG control or miR-125b–overexpressing BMCs were cultured in 20 ng/mL GMCSF to induce differentiation into dendritic cells. The number of dendritic cells was determined by flow cytometry 6 days after culture. (E) MG or miR-125b–transduced BMCs were cultured in 50 ng/mL SCF. When the miR-125b–overexpressing cells were confluent between 4 and 8 days after plating, the cells were reseeded at a starting density of 20K per mL. The x-axis represents the passage number, and the y-axis represents the cell density. Panels A through E are representative of at least 2 independent experiments. (F) MiR-125b–overexpressing BMCs were cultured and expanded in vitro. One million GFP+ cells were injected into sublethally irradiated C57bl/6 mice. Two months posttransplantation, recipient blood was subjected to flow cytometric analysis to determine engraftment of GFP+ cells. The pictures of (G) spleen and (H) femur are representative examples of these organs taken from moribund miR-125b–transduced mice (right panel) and age-matched C57bl/6 controls (left panel). Representative of 6 mice.
Figure 3
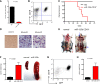
Effect of overexpressing miR-125b in HSPCs and MPs. (A) Equal numbers of MG or MG-125b–transduced Lin−cKit+Sca1+ HSPCs were cultured, and the cell density was determined using flow cytometry. Two-way analysis of variance was used to obtain P values. The number of (B) Lin−cKit+Sca1+ HSPCs and (C) Lin−cKit+Sca1− MPs was determined by flow cytometry 6 days after culture. (D) The ratio of MPs to HSPCs was calculated and plotted. Two independent experiments were performed. (E) Equal number of MG or MG-125b–transduced Lin−cKit+Sca1− MPs were cultured. The cell density was determined according to the indicated time. Representative of two independent experiments. (F) The density of myeloids (Cd11b+), granulocytes (GR1+), or nongranulocytic myeloids (CD11b+GR1−) cells after growing miR-125b–overexpressing MPs was determined by flow cytometry 10 days later. Two independent experiments performed. (G) Sorted MG or miR-125b–transduced Lin−cKit+ Sca1− MPs were cultured and passaged similarly as described in the Figure 2E legend. (H) Sublethally irradiated C57bl/6 recipients were injected with common myeloid progenitors sorted from MG-125b mice. Wright stain of the blood was performed when the recipients were moribund. (I) The spleen and (J) femur were harvested and imaged when the mice were moribund and sacrificed. Representative of 4 mice. Control mice represent recipient mice injected with BMCs from MG mice.
Figure 4
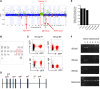
Rescued expression of IRF4 inhibits miR-125b–induced myeloid leukemia. (A) IRF4 inhibits miR-125b–induced hyperproliferation in vitro. Control cells correspond to MG-infected BMCs. BMCs were transduced with retroviruses that encode miR-125b and HcRed. These cells were then transduced with MG or MIG-IRF4 retroviruses, which coexpresses GFP and IRF4. HcRed+GFP+ cells were sorted, and 20K cells per mL of cells were plated. The cell number was determined 3 days later. Representative of 3 experiments. (B) BMCs were transduced with retroviruses that encode miR-125b and mCherry. These cells were then infected with MG or MIG-IRF4 viruses. Shown are flow cytometric plots of these cells and infection efficiency before transplantation into mice. Plots show cells overexpressing miR-125b in the x-axis (mCherry+) and either GFP+ or GFP+ IRF4-overexpressing cells in the y-axis. (C) BMCs described in panel B were transplanted into recipient C57bl/6 mice. One month after transplantation, the peripheral blood of the recipient mice was analyzed by flow cytometry. The left and right panels represent the blood of recipient mice transplanted with BMCs coinfected with miR125b-mCherry with GFP vector and miR125b-mCherry with IRF4-GFP vector, respectively. Representative of 4 mice. (D) Recipient mice were transplanted with donor BMCs that overexpress miR-125b alone or along with restored IRF4 expression mice. The peripheral blood of the recipient mice (>6 mice per group) was analyzed by flow cytometry. Plot displays the Cd11b+ myeloid cell count 3 months after transplantation of donor cells. Control mice represent normal healthy C57bl/6 mice. P value obtained through Mann-Whitney t test. (E) The percent splenic Cd11b+ myeloid was determined using flow cytometry ∼3 months after cell transplantation. (F) Representative images of the femur and tibia of the corresponding recipient mice are shown.
Figure 5
MiR-125b induces tumorigenesis in pre-B cells. (A) MG and MG-125b were bled 12 weeks after bone marrow reconstitution. The figure represents the percent of B cells (CD19+) within the GFP+ gated population in the peripheral blood of these mice (at least 3 mice per group). P value was calculated by Student t test. (B) GFP+CD19+ B cells were sorted from MG-125b mice 4 to 6 months after bone marrow reconstitution. CD19+ B cells (35-52K) were transplanted into sublethally irradiated mice. The figure shows the flow cytometric analysis of the secondary recipient mice (bone marrow) 6 weeks after transplantation. Representative of 8 mice. (C) The figure shows the survival of curve of the secondary recipient mice transplanted with GFP+CD19+ cells from MG-125b mice (8 mice) or total BMCs from MG mice (6 mice). (D) Wright stain was performed from the blood of recipient mice injected with miR-125b–overexpressing CD19+ cells. The dark purple cells represent leukocytes. The smaller cells with central pallor are red blood cells. (E) Some recipients of miR-125b–overexpressing CD19+ cells develop lymphomas. Lymphomas shown at the superficial cervical (top blue arrow) and inguinal lymph node sites (side blue arrow). The normal mouse shown is a healthy C57bl/6 mouse. (F) Left panel, The spleen weight of the recipient mice transplanted with GFP+CD19+ cells (denoted as “miR-125b CD19+”) from MG-125b mice or total BMCs from MG animals were obtained 4 to 6 months after transplantation. Six mice per group. Right panel, Representative images of spleens harvested from normal C57bl/6 control and miR-125b CD19 transplanted mice 6 months posttransplant. (G) MiR-125b induces pre-B-cell cancer. Sorted GFP+CD19+ cells (10K) harvested from mice reconstituted with miR-125b–overexpressing EμMT BMCs were injected into sublethally irradiated secondary C57bl/6 recipients. Bone marrow of the secondary recipient harvested when the mice became moribund, and figure shows flow cytometric plot of the leukocyte population of the bone marrow. (H) Spleen weights of the secondary recipients described in panel H are shown (4 mice). The controls signify spleen weight from secondary recipients injected with cells from MG reconstituted mice (8 mice). P value was calculated by Student t test.
Figure 6
Deletion of IRF4 in miR-125b cells. (A) Analysis of genetic mutations in cancer B cells through comparative genomic hybridization microarray (aCGH). Genomic DNA harvested from sorted miR-125b–overexpressing GFP+CD19+ cancer B cells was subjected to aCGH analysis. Genomic DNA from normal C57bl/6 mice of the same gender (female) was used as controls. The figure represents the relative amount of genetic content of the cancer sample vs the C57bl/6 control (plotted as ratio in y-axis). A genomic region was considered different between the samples if 3 consecutive probes exhibited different signal intensities in the microarray. Regions highlighted in green and orange indicate genomic areas in which the cancer cells have higher and lower DNA content, respectively. The other aCGH analysis is displayed in supplemental Figure 6A. (B) G-band karyotyping of miR-125b–induced cancer B cells. Trisomy 11 is highlighted in red box. (C) Flow cytometric analysis of cancer B cells. Sorted GFP+CD19+ cells isolated from independent groups of MG-125b mice were transplanted into recipient mice. Upon cancer development, the bone marrow of these mice (highlight in red) was analyzed by flow cytometry. The plot shows the samples within the CD19+ gated population. The sample overlaid in black represents total BMCs harvested from healthy control C57bl/6 mice. Group 1 and group 2 corresponds to the aCGH samples displayed in panel A and supplemental Figure 6A, respectively. (D) IRF4 locus and genotyping primer sequences. IRF4 locus is shown with exons represented as solid bars. “Start,” “ex,” and “int” signifies translation start site, exon, and intron, respectively. The red bars represent the PCR product amplified by the primers used for genotyping in panel E-F and supplemental Figure 6E. (E) Quantitative PCR analysis of IRF4 locus. The relative amount of genomic DNA from miR-125b–induced cancer B cells were quantified by quantitative PCR and normalized to control Hsp70 locus. The control samples are genomic DNA harvested from normal C57bl/6 mice. The PCR amplifies a region spanning the translational start site (denoted as “start” in panel C). (F) Genomic DNA from miR-125b–induced cancer B cells was subjected to genotyping analysis. Exon2 (ex2), exon4 (ex4), and exon6 (ex6) of IRF4 were assessed. Bach1 locus was used as positive control. Samples 3-4 and 5-6 correspond to miR-125b–induced cancer B cells harvested from mice originating from group 1 and group 2 described in Figure 5G, respectively. The agarose gel images of the PCRs are displayed.
Similar articles
- MicroRNA-125b transforms myeloid cell lines by repressing multiple mRNA.
Bousquet M, Nguyen D, Chen C, Shields L, Lodish HF. Bousquet M, et al. Haematologica. 2012 Nov;97(11):1713-21. doi: 10.3324/haematol.2011.061515. Epub 2012 Jun 11. Haematologica. 2012. PMID: 22689670 Free PMC article. - Oncomir miR-125b regulates hematopoiesis by targeting the gene Lin28A.
Chaudhuri AA, So AY, Mehta A, Minisandram A, Sinha N, Jonsson VD, Rao DS, O'Connell RM, Baltimore D. Chaudhuri AA, et al. Proc Natl Acad Sci U S A. 2012 Mar 13;109(11):4233-8. doi: 10.1073/pnas.1200677109. Epub 2012 Feb 24. Proc Natl Acad Sci U S A. 2012. PMID: 22366319 Free PMC article. - Epigenetic silencing of miR-125b is required for normal B-cell development.
Li G, So AY, Sookram R, Wong S, Wang JK, Ouyang Y, He P, Su Y, Casellas R, Baltimore D. Li G, et al. Blood. 2018 Apr 26;131(17):1920-1930. doi: 10.1182/blood-2018-01-824540. Epub 2018 Mar 19. Blood. 2018. PMID: 29555645 Free PMC article. - MicroRNA21 and the various types of myeloid leukemia.
Panagal M, S R SK, P S, M B, M K, Gopinathe V, Sivakumare P, Sekar D. Panagal M, et al. Cancer Gene Ther. 2018 Aug;25(7-8):161-166. doi: 10.1038/s41417-018-0025-2. Epub 2018 May 25. Cancer Gene Ther. 2018. PMID: 29795410 Review. - MicroRNAs expressed in hematopoietic stem/progenitor cells are deregulated in acute myeloid leukemias.
Testa U, Pelosi E. Testa U, et al. Leuk Lymphoma. 2015 May;56(5):1466-74. doi: 10.3109/10428194.2014.955019. Epub 2015 Jan 21. Leuk Lymphoma. 2015. PMID: 25242094 Review.
Cited by
- Long non-coding RNA LINC01018 inhibits the progression of acute myeloid leukemia by targeting miR-499a-5p to regulate PDCD4.
Zhou H, Shi P, Jia X, Xue Q. Zhou H, et al. Oncol Lett. 2021 Jul;22(1):541. doi: 10.3892/ol.2021.12802. Epub 2021 May 20. Oncol Lett. 2021. PMID: 34079594 Free PMC article. - The miR-223 host non-coding transcript linc-223 induces IRF4 expression in acute myeloid leukemia by acting as a competing endogenous RNA.
Mangiavacchi A, Sorci M, Masciarelli S, Larivera S, Legnini I, Iosue I, Bozzoni I, Fazi F, Fatica A. Mangiavacchi A, et al. Oncotarget. 2016 Sep 13;7(37):60155-60168. doi: 10.18632/oncotarget.11165. Oncotarget. 2016. PMID: 27517498 Free PMC article. - B-cell acute lymphoblastic leukemia-related microRNAs: uncovering their diverse and special roles.
Lv M, Zhu S, Peng H, Cheng Z, Zhang G, Wang Z. Lv M, et al. Am J Cancer Res. 2021 Apr 15;11(4):1104-1120. eCollection 2021. Am J Cancer Res. 2021. PMID: 33948348 Free PMC article. Review. - MicroRNAs as regulatory elements in immune system logic.
Mehta A, Baltimore D. Mehta A, et al. Nat Rev Immunol. 2016 Apr 28;16(5):279-94. doi: 10.1038/nri.2016.40. Nat Rev Immunol. 2016. PMID: 27121651 Review. - Dexmedetomidine-up-regulated microRNA-381 exerts anti-inflammatory effects in rats with cerebral ischaemic injury via the transcriptional factor IRF4.
Fang H, Li HF, Yan JY, Yang M, Zhang JP. Fang H, et al. J Cell Mol Med. 2021 Feb;25(4):2098-2109. doi: 10.1111/jcmm.16153. Epub 2020 Dec 13. J Cell Mol Med. 2021. PMID: 33314611 Free PMC article.
References
- Chapiro E, Russell LJ, Struski S, et al. A new recurrent translocation t(11;14)(q24;q32) involving IGH@ and miR-125b-1 in B-cell progenitor acute lymphoblastic leukemia. Leukemia. 2010;24(7):1362–1364. - PubMed
- Tassano E, Acquila M, Tavella E, Micalizzi C, Panarello C, Morerio C. MicroRNA-125b-1 and BLID upregulation resulting from a novel IGH translocation in childhood B-Cell precursor acute lymphoblastic leukemia. Genes Chromosomes Cancer. 2010;49(8):682–687. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 1R01AI079243/AI/NIAID NIH HHS/United States
- K99HL118754/HL/NHLBI NIH HHS/United States
- K99 HL118754/HL/NHLBI NIH HHS/United States
- 1F32 CA139883-01A1/CA/NCI NIH HHS/United States
- R01 AI093531/AI/NIAID NIH HHS/United States
- 1R01AI093531/AI/NIAID NIH HHS/United States
- R01 AI079243/AI/NIAID NIH HHS/United States
- F32 CA139883/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials