Pre-administration of G9a/GLP inhibitor during synaptogenesis prevents postnatal ethanol-induced LTP deficits and neurobehavioral abnormalities in adult mice - PubMed (original) (raw)
Pre-administration of G9a/GLP inhibitor during synaptogenesis prevents postnatal ethanol-induced LTP deficits and neurobehavioral abnormalities in adult mice
Shivakumar Subbanna et al. Exp Neurol. 2014 Nov.
Abstract
It has been widely accepted that deficits in neuronal plasticity underlie the cognitive abnormalities observed in fetal alcohol spectrum disorder (FASD). Exposure of rodents to acute ethanol on postnatal day 7 (P7), which is equivalent to the third trimester of fetal development in human, induces long-term potentiation (LTP) and memory deficits in adult animals. However, the molecular mechanisms underlying these deficits are not well understood. Recently, we found that histone H3 dimethylation (H3K9me2), which is mediated by G9a (lysine dimethyltransferase), is responsible for the neurodegeneration caused by ethanol exposure in P7 mice. In addition, pharmacological inhibition of G9a prior to ethanol treatment at P7 normalized H3K9me2 proteins to basal levels and prevented neurodegeneration in neonatal mice. Here, we tested the hypothesis that pre-administration of G9a/GLP inhibitor (Bix-01294, Bix) in conditions in which ethanol induces neurodegeneration would be neuroprotective against P7 ethanol-induced deficits in LTP, memory and social recognition behavior in adult mice. Ethanol treatment at P7 induces deficits in LTP, memory and social recognition in adult mice and these deficits were prevented by Bix pretreatment at P7. Together, these findings provide physiological and behavioral evidence that the long-term harmful consequences on brain function after ethanol exposure with a third trimester equivalent have an epigenetic origin.
Keywords: Bix; Epigenetics; Ethanol; FASD; H3K9; Histones; Memory loss; Methyltransferase; Postnatal development; Synaptic plasticity.
Published by Elsevier Inc.
Conflict of interest statement
Disclosure
The authors declare no conflict of interest.
Figures
Fig. 1
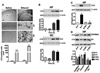
Ethanol induces apoptotic neurodegeneration in the P7 mouse brain and pharmacological inhibition of G9a rescues P7 ethanol-induced neurodegeneration and H3K9me2 in the neonatal mouse brain. (A) Coronal brain sections (hippocampus and retrosplenial cortex) from saline- and ethanol-treated animals were immunostained with an anti-rabbit CC3 antibody. The white arrows indicate the CC3-positive neurons in the hippocampus and retrosplenial cortex. Scale bars = 200 µm. The respective images were enlarged to show the CC3-positive cells (*). The scale bars represent 50 µm. CC3-positive cells were quantified in the hippocampus and retrosplenial cortex (n = 10 pups/group). Student’s t test: ***p < 0.001 vs. respective saline group . Each point is presented as the mean ± SEM. (B) Western blot analysis of CC3 using cytosolic extracts (20 µg) of hippocampal and cortical samples from the saline- and ethanol-treated groups (n = 8 pups/group). The graphs represent the ratio of the proteins normalized to the expression of β-actin. ***p < 0.001 vs. 0 h (respective saline control) . Each point is presented as the mean ± SEM. (C and D) Mice pre-treated (30 min) with Bix (1 mg/kg) or vehicle were exposed to ethanol, and hippocampal extracts from S + V, E + V, S + Bix and E + Bix (n = 6 pups/group) were collected 8 h after first dose of ethanol treatment and processed for Western blotting to analyze CC3, H3K9me2 and H3 levels. β-actin was used as a loading control. Representative blots are shown for the hippocampal cytosolic (CC3) and nuclear (H3K9 and H3) extracts. HP, hippocampus; NC, neocortex. Each point is presented as the mean ± SEM. One-way ANOVA with Bonferroni’s post hoc tests; ***p < 0.001, **p < 0.01 vs. S + V; #p < 0.001 vs. E + V .
Fig. 2
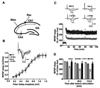
Inhibition of G9a before ethanol treatment in P7 pups prevents long-lasting synaptic deficits in adult mice. (A) A schematic diagram showing the stimulating and recording electrode positions in the CA1 region of the hippocampus. (B) A summary graph showing the field input/output relationships for P7 treated S + V, E + V, S + Bix and E + Bix adult mice.Insert: An example of traces taken from representative experiments from input/output relationships for S + V. Although not shown other groups also exhibited similar pattern . (C) Time course of the averages of the fEPSP slopes in slices obtained from S+V, E+V, S + Bix- and E + Bix-treated mice. The fEPSP slopes were normalized to the average value 10 min before stimulation in each experiment. Arrows denote the time of tetanic stimulation (4 pulses at 100 Hz, with bursts repeated at 5 Hz, and each tetanus including three 10-burst trains separated by 15 s). Representative traces of fEPSPs before (trace 1) and after (trace 2) induction of LTP in hippocampal slices from S +V, E + V, S + Bix and E + Bix mice . (D) A combined plot of the averages of the fEPSP slopes at several time points. Each point is presented as the mean ± SEM (n= 5 mice/group; 10 slices/group). Two-way ANOVA with Bonferroni’s post hoc tests; ***p < 0.001 vs. S + V; #p < 0.001 vs. E + V .
Fig. 3
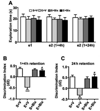
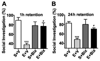
Inactivation of G9a activity before ethanol treatment at P7 prevents object recognition memory loss in adult mice. (A) Level of exploration was measured at el and e2, respectively: the time spent exploring the two objects in T1 and T2 (at 1+4 and 24 h) by S+V, E+V, S+ Bix, and E+ Bix-treated mice. (B) Discrimination indices (d2) obtained from the S+V, E+V, S+ Bix, and E+ Bix-treated mice after 1and 4 h retention intervals. (C) Discrimination indices (d2) obtained from the S+V, E+V, S+ Bix, and E+ Bix-treated mice after 24 h retention intervals. Each point is the mean + SEM (n= 8 mice/group). One-way ANOVA with Bonferroni’s post hoc test; ***p < 0.001 vs. S + V; #p < 0.001 vs. E + V .
Fig. 4
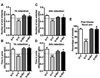
P7 ethanol treatment impairs and prior administration of G9a/GLP inhibitor prevents the spontaneous alternation performance deficit in adult mice. (A) Total number of arm entries reflecting exploratory activities of mice in the Y-maze does not differ between the four groups (p > 0.05). (B) The time spent in each arm was not different between four groups (p > 0.05). (C) The spontaneous alternation performance was reduced in by ethanol (E+V) and was rescued by Bix treatment (E+ Bix). Alternation performance was not affected by saline (S+V) and Bix (S+Bix) treatment. Each point is the mean ± SEM (n= 8 mice/group). (***p < 0.001 vs. S+V; #p < 0.001 vs. E+V) . One-way ANOVA with Bonferroni’s post hoc test.
Fig. 5
P7 ethanol treatment impairs and Bix pretreatment rescues impaired spatial memory performance as measured by Y maze. (A–D) Discrimination ratio [preference for the Novel arm over the familiar Other arm (Novel/Novel + Other)] for arm entries (A and C, 1 h and 24 h) and dwell time (B and D, 1 h and 24 h) of S+V and E+V mice treated with or without Bix (S+Bix and E+Bix), 1 or 24 h after the first encounter with the partially opened maze. The dashed line indicates chance performance (0.5). (E) The percentage of animals selecting the novel arm as the first choice is shown for S+V and E+V mice treated with or without Bix (S+Bix and E+Bix), 1 and 24 h after the first encounter with the partially opened maze. Each point is the mean + SEM (n= 8 mice/group). Two-way ANOVA with Bonferroni’s post hoc test; ***p < 0.001 vs. S + V; # p < 0.01 vs. E + V .
Fig. 6
Bix pretreatment rescues P7 ethanol-induced social recognition memory loss in the adult mice. (A and B) Percent of social investigation is shown for S+V, E+V, S+ Bix, and E+ Bix-treated mice, 1 (A) and 24 h (B) after the first encounter with same juvenile mice. Each point is the mean ± SEM (n= 8 mice/group). Two-way ANOVA with Bonferroni’s _post hoc_test. ***p < 0.001 vs. S + V; # p < 0.01 vs. E + V .
Similar articles
- Ethanol induced acetylation of histone at G9a exon1 and G9a-mediated histone H3 dimethylation leads to neurodegeneration in neonatal mice.
Subbanna S, Nagre NN, Shivakumar M, Umapathy NS, Psychoyos D, Basavarajappa BS. Subbanna S, et al. Neuroscience. 2014 Jan 31;258:422-32. doi: 10.1016/j.neuroscience.2013.11.043. Epub 2013 Dec 1. Neuroscience. 2014. PMID: 24300108 Free PMC article. - G9a-mediated histone methylation regulates ethanol-induced neurodegeneration in the neonatal mouse brain.
Subbanna S, Shivakumar M, Umapathy NS, Saito M, Mohan PS, Kumar A, Nixon RA, Verin AD, Psychoyos D, Basavarajappa BS. Subbanna S, et al. Neurobiol Dis. 2013 Jun;54:475-85. doi: 10.1016/j.nbd.2013.01.022. Epub 2013 Feb 8. Neurobiol Dis. 2013. PMID: 23396011 Free PMC article. - Ethanol exposure induces neonatal neurodegeneration by enhancing CB1R Exon1 histone H4K8 acetylation and up-regulating CB1R function causing neurobehavioral abnormalities in adult mice.
Subbanna S, Nagre NN, Umapathy NS, Pace BS, Basavarajappa BS. Subbanna S, et al. Int J Neuropsychopharmacol. 2014 Oct 31;18(5):pyu028. doi: 10.1093/ijnp/pyu028. Int J Neuropsychopharmacol. 2014. PMID: 25609594 Free PMC article. - Epigenetics and memory: Emerging role of histone lysine methyltransferase G9a/GLP complex as bidirectional regulator of synaptic plasticity.
Pang KKL, Sharma M, Sajikumar S. Pang KKL, et al. Neurobiol Learn Mem. 2019 Mar;159:1-5. doi: 10.1016/j.nlm.2019.01.013. Epub 2019 Jan 28. Neurobiol Learn Mem. 2019. PMID: 30703547 Review. - Mechanisms Underlying Memory Impairment Induced by Fructose.
Franco-Pérez J. Franco-Pérez J. Neuroscience. 2024 Jun 7;548:27-38. doi: 10.1016/j.neuroscience.2024.04.001. Epub 2024 Apr 26. Neuroscience. 2024. PMID: 38679409 Review.
Cited by
- Epigenetic Modifications, Alcoholic Brain and Potential Drug Targets.
Jangra A, Sriram CS, Pandey S, Choubey P, Rajput P, Saroha B, Bezbaruah BK, Lahkar M. Jangra A, et al. Ann Neurosci. 2016 Oct;23(4):246-260. doi: 10.1159/000449486. Epub 2016 Oct 4. Ann Neurosci. 2016. PMID: 27780992 Free PMC article. Review. - Oxidative stress-induced aberrant G9a activation disturbs RE-1-containing neuron-specific genes expression, leading to degeneration in human SH-SY5Y neuroblastoma cells.
Kim HT, Ohn T, Jeong SG, Song A, Jang CH, Cho GW. Kim HT, et al. Korean J Physiol Pharmacol. 2021 Jan 1;25(1):51-58. doi: 10.4196/kjpp.2021.25.1.51. Korean J Physiol Pharmacol. 2021. PMID: 33361537 Free PMC article. - Mechanisms Underlying the Regulation of HP1γ by the NGF-PKA Signaling Pathway.
Seo S, Mathison A, Grzenda A, Podratz J, Calvo E, Brimijoin S, Windebank A, Iovanna J, Lomberk G, Urrutia R. Seo S, et al. Sci Rep. 2018 Oct 10;8(1):15077. doi: 10.1038/s41598-018-33475-y. Sci Rep. 2018. PMID: 30305677 Free PMC article. - CB1R regulates CDK5 signaling and epigenetically controls Rac1 expression contributing to neurobehavioral abnormalities in mice postnatally exposed to ethanol.
Joshi V, Subbanna S, Shivakumar M, Basavarajappa BS. Joshi V, et al. Neuropsychopharmacology. 2019 Feb;44(3):514-525. doi: 10.1038/s41386-018-0181-y. Epub 2018 Aug 22. Neuropsychopharmacology. 2019. PMID: 30143782 Free PMC article. - Ethanol sustains phosphorylated tau protein in the cultured neonatal rat hippocampus: Implications for fetal alcohol spectrum disorders.
Bailey CS, Jagielo-Miller JE, Keller PS, Glaser EP, Wilcox AL, Prendergast MA. Bailey CS, et al. Alcohol. 2022 Sep;103:45-54. doi: 10.1016/j.alcohol.2022.07.007. Epub 2022 Aug 12. Alcohol. 2022. PMID: 35964913 Free PMC article.
References
- Abdollah S, Catlin MC, Brien JF. Ethanol neuro-behavioural teratogenesis in the guinea pig: behavioural dysfunction and hippocampal morphologic change. Can J Physiol Pharmacol. 1993;71:776–782. - PubMed
- Alvarez P, Wendelken L, Eichenbaum H. Hippocampal formation lesions impair performance in an odor-odor association task independently of spatial context. Neurobiol Learn Mem. 2002;78:470–476. - PubMed
- APA. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatry Association Press; 2000.
- Balemans MC, Ansar M, Oudakker AR, van Caam AP, Bakker B, Vitters EL, van der Kraan PM, de Bruijn DR, Janssen SM, Kuipers AJ, Huibers MM, Maliepaard EM, Walboomers XF, Benevento M, Nadif Kasri N, Kleefstra T, Zhou H, Van der Zee CE, van Bokhoven H. Reduced Euchromatin histone methyltransferase 1 causes developmental delay, hypotonia, and cranial abnormalities associated with increased bone gene expression in Kleefstra syndrome mice. Dev Biol. 2014;386:395–407. - PubMed
- Baron-Cohen S, Ring H, Moriarty J, Schmitz B, Costa D, Ell P. Recognition of mental state terms. Clinical findings in children with autism and a functional neuroimaging study of normal adults. British Journal of Psychiatry. 1994;165:640–649. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical