Neuroinflammation and neurodegeneration in adult rat brain from binge ethanol exposure: abrogation by docosahexaenoic acid - PubMed (original) (raw)
Neuroinflammation and neurodegeneration in adult rat brain from binge ethanol exposure: abrogation by docosahexaenoic acid
Nuzhath Tajuddin et al. PLoS One. 2014.
Abstract
Evidence that brain edema and aquaporin-4 (AQP4) water channels have roles in experimental binge ethanol-induced neurodegeneration has stimulated interest in swelling/edema-linked neuroinflammatory pathways leading to oxidative stress. We report here that neurotoxic binge ethanol exposure produces comparable significant effects in vivo and in vitro on adult rat brain levels of AQP4 as well as neuroinflammation-linked enzymes: key phospholipase A2 (PLA2) family members and poly (ADP-ribose) polymerase-1 (PARP-1). In adult male rats, repetitive ethanol intoxication (3 gavages/d for 4 d, ∼ 9 g/kg/d, achieving blood ethanol levels ∼ 375 mg/dl; "Majchrowicz" model) significantly increased AQP4, Ca+2-dependent PLA2 GIVA (cPLA2), phospho-cPLA2 GIVA (p-cPLA2), secretory PLA2 GIIA (sPLA2) and PARP-1 in regions incurring extensive neurodegeneration in this model--hippocampus, entorhinal cortex, and olfactory bulb--but not in two regions typically lacking neurodamage, frontal cortex and cerebellum. Also, ethanol reduced hippocampal Ca+2-independent PLA2 GVIA (iPLA2) levels and increased brain "oxidative stress footprints" (4-hydroxynonenal-adducted proteins). For in vitro studies, organotypic cultures of rat hippocampal-entorhinocortical slices of adult age (∼ 60 d) were ethanol-binged (100 mM or ∼ 450 mg/dl) for 4 d, which augments AQP4 and causes neurodegeneration (Collins et al. 2013). Reproducing the in vivo results, cPLA2, p-cPLA2, sPLA2 and PARP-1 were significantly elevated while iPLA2 was decreased. Furthermore, supplementation with docosahexaenoic acid (DHA; 22:6n-3), known to quell AQP4 and neurodegeneration in ethanol-treated slices, blocked PARP-1 and PLA2 changes while counteracting endogenous DHA reduction and increases in oxidative stress footprints (3-nitrotyrosinated proteins). Notably, the PARP-1 inhibitor PJ-34 suppressed binge ethanol-dependent neurodegeneration, indicating PARP upstream involvement. The results with corresponding models support involvement of AQP4- and PLA2-associated neuroinflammatory pro-oxidative pathways in the neurodamage, with potential regulation by PARP-1 as well. Furthermore, DHA emerges as an effective inhibitor of these binge ethanol-dependent neuroinflammatory pathways as well as associated neurodegeneration in adult-age brain.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
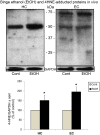
Figure 1. Effects of binge ethanol exposure on oxidative stress (4HNE-) protein footprint levels in vivo.
Significant increases over respective controls (Cont) in levels of oxidative stress footprint, 4-hydroxynonenal (4HNE)-adducted proteins, in hippocampus (HC) and entorhinal cortex (EC) following neurotoxic binge ethanol treatment of adult male rats for 4 days (Majchrowicz model). Top: representative immunoblots of 4HNE-adducted proteins in HC and EC. Bottom: quantitation of immunoblots of 4HNE-adducted proteins in HC and EC. *p<0.01 vs. control (Cont); n = 4–7 rats per group.
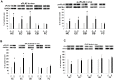
Figure 2. Selective effects of binge ethanol exposure on brain PLA2 levels in vivo.
Significant alterations compared to respective controls (C) in protein expression (immunoblots) of PLA2 enzymes in brain regions selectively incurring neurodamage due to binge ethanol (E) treatment in adult male rats for 4 days (Majchrowicz model).
Fig. 2A
: Significantly increased levels of cPLA2 (top) and p-cPLA2 (bottom) in the hippocampus (HC), entorhinal cortex (EC) and olfactory bulb (OB), but not in cerebellum (CB) and frontal cortex (FC).
Fig. 2B
: Significantly increased sPLA2 levels in the HC, EC and OB, but not in CB and FC.
Fig. 2C
. Significantly decreased levels of iPLA2 in the HC. *p<0.05 vs. C. **p<0.01 vs. C. n = 4–7 rats/group.
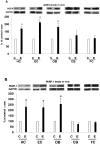
Figure 3. Selective effects of binge ethanol exposure on brain AQP4 and PARP-1 levels in vivo.
Significant elevations over respective controls (C) in levels of AQP4 and PARP-1 in those brain regions selectively incurring neurodamage due to binge ethanol (E) treatment in adult male rats for 4 days (Majchrowicz model).
Fig. 3A
: Significantly increased AQP4 levels in the HC, EC and OB, but not in CB and FC.
Fig. 3B
: Significantly increased PARP-1 levels in the HC, EC and OB, but not in CB and FC. *p<0.05 vs. C. n = 4–7 rats/group.
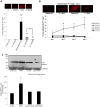
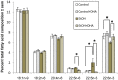
Figure 4. Effects of binge ethanol treatment and DHA supplementation on neurodegeneration and oxidative (3NT-) protein footprints in adult-age HEC slice cultures.
Binge ethanol treatment of adult-age rat HEC slice cultures for 4 days as described in Methods significantly increases degenerating neurons (PI staining) and the levels of oxidative stress footprints (3NT-proteins), and supplementation of cultures with DHA prevents the increases.
Fig. 4A
: (top) Representative PI-stained images showing increased neurodegeneration after 3 days of binge ethanol treatment (100 mM), with DHA supplementation (25 µM) suppressing PI staining (neurodegeneration). (Bottom) Quantitation of PI labeling shows significant neurodegeneration due to 4 days of binge ethanol exposure (E), and neuroprotection against E by DHA at 25 and 50 µM. **p<0.01 vs. C. #p<0.05 vs. E.
Fig. 4B
. Quantitation of PI labeling reveals significantly increased neurodegeneration over control due to binge E as early as 2 days of binge ethanol treatment, with prevention of the neurodegeneration throughout the 4 days of treatment by DHA supplementation. *p<0.05 vs. control (C) or E+DHA.
Fig. 4C
(Top) Representative immunoblots of 3NT-proteins in HEC slice cultures following binge ethanol exposure (100 mM) for 4 days. (Bottom) Quantitation of immunoblots showing that binge ethanol exposure causes increased 3NT-proteins in the HEC slice cultures, and DHA supplementation (25 µM) prevents the increases. *p<0.05 vs. Control.
Figure 5. Effects of binge ethanol treatment and DHA supplementation on PLA2 and PARP-1 levels in adult-age HEC slice cultures.
Significant neuroinflammatory enzyme alterations compared to control (C) caused by 4 days of binge ethanol (E) treatment (100 mM) of adult-age rat HEC slice cultures, and prevention of the alterations by supplementation of cultures with DHA (25 µM).
Fig. 5A
: Increased levels of cPLA2 GIVA over control levels due to binge E and inhibition of the increase by DHA supplementation (E+ DHA). *p<0.05 vs. C; #p<0.05 vs. E.
Fig. 5B
: Increased levels of p-cPLA2 GIVA over control levels due to binge E and inhibition of the increase by DHA supplementation (E+DHA). *p<0.05 vs. C; #p<0.05 vs. E.
Fig. 5C
: Increased levels of sPLA2 GIIA over control levels due to binge E and inhibition of the increase by DHA supplementation (E+DHA). *p<0.05 vs. C; #p<0.05 vs. E.
Fig. 5D
: Reduced levels of iPLA2 GVIA with respect to control levels due to binge E and partial blockade of the reduction by DHA supplementation (E+DHA). *p<0.05 vs. C.
Fig. 5E
: Increased levels of PARP-1 over control levels due to binge E and inhibition of the increase by DHA supplementation (E+DHA). *p<0.05 vs. C; #p<0.05 vs. E.
Figure 6. Effect of binge ethanol treatment and DHA supplementation on contents of DHA and selected unsaturated fatty acids in adult-age HEC slice cultures.
Binge ethanol treatment (EtOH, 100 mM) of adult-age rat HEC slice cultures for 4 days as described in Methods significantly decreases the slice content of endogenous DHA (22:6n−3); supplementation of ethanol-binged cultures throughout with 25 µM DHA normalizes the DHA content. Binge ethanol treatment also increased the endogenous content of 22:5n−3, and DHA supplementation reduced the levels of this potential DHA precursor, as well as reduced the endogenous levels of ARA (22:4n−6). All other unsaturated fatty acids (not shown) were not significantly changed by binge ethanol treatment and DHA supplementation. *p<0.05 (n = 3/group, Tukey's t-test).
Figure 7. Effect of PARP-1 inhibitor PJ-34 on ethanol-induced neurodegeneration in adult-age HEC slice cultures.
Neurodegeneration (PI staining) due to 4 days of binge ethanol treatment (100 mM) of rat adult-age organotypic HEC slice cultures is suppressed by co-treatment with PARP-1 inhibitor, PJ-34 (10 µM). (Top) Images of representative PI-stained slices from C, PJ-34, E and E+PJ-34 slice cultures indicate increased neurodegeneration (E) that is reduced in E+PJ-34. (Bottom) Quantitation of PI staining demonstrates increased neurodegeneration due to binge ethanol (E) that is significantly suppressed by PJ-34 (E + PJ-34). **p<0.01 vs. C. ##p<0.01 vs. E.
Similar articles
- Effect of repetitive daily ethanol intoxication on adult rat brain: significant changes in phospholipase A2 enzyme levels in association with increased PARP-1 indicate neuroinflammatory pathway activation.
Tajuddin NF, Przybycien-Szymanska MM, Pak TR, Neafsey EJ, Collins MA. Tajuddin NF, et al. Alcohol. 2013 Feb;47(1):39-45. doi: 10.1016/j.alcohol.2012.09.003. Epub 2012 Oct 25. Alcohol. 2013. PMID: 23102656 Free PMC article. - Phospholipase A2, oxidative stress, and neurodegeneration in binge ethanol-treated organotypic slice cultures of developing rat brain.
Moon KH, Tajuddin N, Brown J 3rd, Neafsey EJ, Kim HY, Collins MA. Moon KH, et al. Alcohol Clin Exp Res. 2014 Jan;38(1):161-9. doi: 10.1111/acer.12221. Epub 2013 Aug 1. Alcohol Clin Exp Res. 2014. PMID: 23909864 Free PMC article. - Alcohol, phospholipase A2-associated neuroinflammation, and ω3 docosahexaenoic acid protection.
Collins MA, Tajuddin N, Moon KH, Kim HY, Nixon K, Neafsey EJ. Collins MA, et al. Mol Neurobiol. 2014 Aug;50(1):239-45. doi: 10.1007/s12035-014-8690-0. Epub 2014 Apr 6. Mol Neurobiol. 2014. PMID: 24705861 Free PMC article. Review. - PARP Inhibition Prevents Ethanol-Induced Neuroinflammatory Signaling and Neurodegeneration in Rat Adult-Age Brain Slice Cultures.
Tajuddin N, Kim HY, Collins MA. Tajuddin N, et al. J Pharmacol Exp Ther. 2018 Apr;365(1):117-126. doi: 10.1124/jpet.117.245290. Epub 2018 Jan 16. J Pharmacol Exp Ther. 2018. PMID: 29339456 Free PMC article. - Neuroinflammatory pathways in binge alcohol-induced neuronal degeneration: oxidative stress cascade involving aquaporin, brain edema, and phospholipase A2 activation.
Collins MA, Neafsey EJ. Collins MA, et al. Neurotox Res. 2012 Jan;21(1):70-8. doi: 10.1007/s12640-011-9276-5. Epub 2011 Sep 17. Neurotox Res. 2012. PMID: 21927955 Free PMC article. Review.
Cited by
- Investigation of Uncovering Molecular Mechanisms of Alcohol-Induced Female Infertility-A Rational Approach.
Lubau NSA, Chengebroyen N, Subramaniyan V. Lubau NSA, et al. Reprod Sci. 2024 Nov 1. doi: 10.1007/s43032-024-01692-8. Online ahead of print. Reprod Sci. 2024. PMID: 39485609 Review. - Ethanol's impact on the brain: a neurobiological perspective on the mechanisms of memory impairment.
Hedayati-Moghadam M, Razazpour F, Pourfridoni M, Mirzaee F, Baghcheghi Y. Hedayati-Moghadam M, et al. Mol Biol Rep. 2024 Jun 25;51(1):782. doi: 10.1007/s11033-024-09748-3. Mol Biol Rep. 2024. PMID: 38918289 Review. - Artemisinin and its derivatives as promising therapies for autoimmune diseases.
Xie K, Li Z, Zhang Y, Wu H, Zhang T, Wang W. Xie K, et al. Heliyon. 2024 Mar 11;10(7):e27972. doi: 10.1016/j.heliyon.2024.e27972. eCollection 2024 Apr 15. Heliyon. 2024. PMID: 38596057 Free PMC article. Review. - Binge ethanol exposure in advanced age elevates neuroinflammation and early indicators of neurodegeneration and cognitive impairment in female mice.
Anton PE, Rutt LN, Kaufman ML, Busquet N, Kovacs EJ, McCullough RL. Anton PE, et al. Brain Behav Immun. 2024 Feb;116:303-316. doi: 10.1016/j.bbi.2023.12.034. Epub 2023 Dec 25. Brain Behav Immun. 2024. PMID: 38151165 Free PMC article. - Omega-3 Recovers Cannabinoid 1 Receptor Expression in the Adult Mouse Brain after Adolescent Binge Drinking.
Martín-Llorente A, Serrano M, Bonilla-Del Río I, Lekunberri L, Ocerin G, Puente N, Ramos A, Rico-Barrio I, Gerrikagoitia I, Grandes P. Martín-Llorente A, et al. Int J Mol Sci. 2023 Dec 10;24(24):17316. doi: 10.3390/ijms242417316. Int J Mol Sci. 2023. PMID: 38139145 Free PMC article.
References
- Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, et al. (2009) Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 373: 2223–33. - PubMed
- Hunt WA (1993) Are binge drinkers more at risk of developing brain damage? Alcohol 10: 559–61. - PubMed
- Virta JJ, Järvenpää T, Heikkilä K, Perola M, Koskenvuo M, et al. (2010) Midlife alcohol consumption and later risk of cognitive impairment: A twin follow-up study. Journal of Alzheimer's Disease 22: 939–948. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AA016959/AA/NIAAA NIH HHS/United States
- T32 DA016176/DA/NIDA NIH HHS/United States
- U01 AA018279/AA/NIAAA NIH HHS/United States
- R01 AA0016959/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous