Inactivation of TRPM7 kinase activity does not impair its channel function in mice - PubMed (original) (raw)
Inactivation of TRPM7 kinase activity does not impair its channel function in mice
Taku Kaitsuka et al. Sci Rep. 2014.
Abstract
Transient receptor potential (TRP) family channels are involved in sensory pathways and respond to various environmental stimuli. Among the members of this family, TRPM7 is a unique fusion of an ion channel and a C-terminus kinase domain that is highly expressed in immune cells. TRPM7 serves as a key molecule governing cellular Mg(2+) homeostasis in mammals since its channel pore is permeable to Mg(2+) ions and can act as a Mg(2+) influx pathway. However, mechanistic links between its kinase activity and channel function have remained uncertain. In this study, we generated kinase inactive knock-in mutant mice by mutagenesis of a key lysine residue involved in Mg(2+)-ATP binding. These mutant mice were normal in development and general locomotor activity. In peritoneal macrophages isolated from adult animals the basal activity of TRPM7 channels prior to cytoplasmic Mg(2+) depletion was significantly potentiated, while maximal current densities measured after Mg(2+) depletion were unchanged in the absence of detectable kinase function. Serum total Ca(2+) and Mg(2+) levels were not significantly altered in kinase-inactive mutant mice. Our findings suggest that abolishing TRPM7 kinase activity does not impair its channel activity and kinase activity is not essential for regulation of mammalian Mg(2+) homeostasis.
Figures
Figure 1. Generation of TRPM7 kinase-dead knock-in mice.
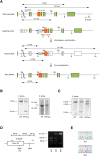
(A) Schematic diagram of the endogenous Trpm7 locus and the targeting vector used to engineer embryonic stem (ES) cells by homologous recombination. This vector contains a point mutation in exon 33 that changes lysine residue at position 1646 to arginine (K1646R). Restriction enzyme sites are indicated (B, BamHI; Bg, BglII; Sc, ScaI). The length of the genomic fragment is also shown when digested by the indicated enzymes. Probes 5′ and 3′ were used to screen ES cells for homologous recombination by Southern blotting. DT-A, diphtheria toxin-A; neo, neomycin-resistant gene. (B) Southern blot analysis of genomic DNA from targeted ES cells using 5′-probe or 3′-probe after digestion with BamHI or ScaI. Full length blots are shown in Fig. S1. (C) Southern blot analysis of genomic DNA from loxP-excised heterozygous mice using 5′-probe after digestion with BglII. Full length blot is shown in Fig. S2. (D) PCR strategy used to genotype the animals. The region surrounding K1646R mutation was amplified by PCR and digested with MseI. A 211 bp fragment undigested at the mutated region is produced from wild-type (WT) allele, while the amplification product from the KR allele is digested into 129 bp and 86 bp fragments at the mutated region. PCR was performed on DNA isolated from tail biopsies; results from WT, TRPM7K/R, and TRPM7R/R animals are shown. Full length gel image is shown in Fig. S3. (E) PCR amplification products from WT and TRPM7R/R animals were subcloned and sequenced to demonstrate the presence of the K1646R mutation; representative traces are shown.
Figure 2. Serum total Mg2+ and Ca2+ levels and general behaviors in TRPM7R/R mice.
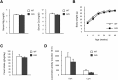
(A) Total Mg2+ and Ca2+ levels in serum obtained from WT and TRPM7R/R mice. Here and below values represent means ± s.e.m. (n = 10–14). (B) Male WT and TRPM7R/R mice were weighed at the indicated times (n = 6). (C,D) General behavior was analyzed in 16 to 18 week-old WT and TRPM7R/R mice. (C) Food intake data. (D) Locomotor activity data (n = 6).
Figure 3. TRPM7 kinase activity in TRPM7R/R mice.
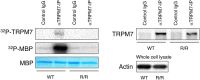
Kinase assay of TRPM7 proteins from WT and TRPMR/R mouse embryonic fibroblasts (MEF). Left panel shows 32P incorporation into autologous TRPM7 and exogenous myelin basic protein (MBP) by TRPM7 immunoprecipitated from WT or TRPM7R/R MEF lysates. Coomassie blue staining of MBP was used to ensure equal quantities of MBP. Right panel shows Western blot analysis of TRPM7 levels in the immunoprecipitates used in the left panel. Probing for actin in the whole cell lysates was used to ensure the presence of equal amount of protein in the lysates before immunoprecipitation. Full length blots and gel images are shown in Fig. S4.
Figure 4. TRPM7 channel and kinase activities in the macrophages of WT and TRPM7R/R mice.
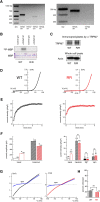
(A) Expression of TRPM7 and TRPM6 mRNA in peritoneal macrophages. RT-PCR analysis of TRPM7 and TRPM6 expression in mouse peritoneal macrophages (left panel) and kidney (right panel). RT, reverse transcriptase. 40 and 38 cycles PCR were used for macrophage and kidney cDNA, respecitvely. Predicted TRPM7 size = 550 bp, predicted TRPM6 size = 475 bp. Full length gel images are shown in Fig. S5. (B) Kinase assay of TRPM7 protein isolated from peritoneal macrophages. 32P incorporation into exogenous MBP by TRPM7 immunoprecipitated from lysates of WT or TRPM7R/R macrophages is shown. Coomassie staining of MBP was used to ensure equal levels of MBP. Full length images are shown in Fig. S6. (C) Western blot analysis of TRPM7 in the immunoprecipitates used in (B). Actin in whole cell lysates was used to ensure the presence of equal protein amounts before immunoprecipitation. Full length gel images are shown in Fig. S7. (D) TRPM7 current-voltage relations recorded in WT (black) and TRPM7R/R (red) peritoneal macrophages. (E) Time course of TRPM7 current development in the same cells. (F) Basal (break-in) and maximum TRPM7 current amplitudes (left) and densities (right) in WT and TRPM7R/R mice. Internal solutions for measurement of basal and maximum currents contained 400 nM free Mg2+ (left). Internal Mg2+ and spermine solutions contained 303 μM Mg2+ or 300 μM spermine with no Mg2+ (F, right). Current amplitudes were obtained from the 125th ramp after break-in, P < 0.01 for basal, P < 0.05 for maximum. Time courses of current development with 300 μM spermine are shown in Fig. S8.(G) Blockade of monovalent TRPM7 current by 10 μM external spermine in macrophages isolated from WT (left) and kinase-dead mutant mice (right). (H) Percent unblocked monovalent TRPM7 current in WT and TRPM7R/R mouse macrophages at −100 mV. Values are means ± s.e.m. Numbers of cells are shown in parentheses.
Similar articles
- Natural and synthetic modulators of SK (K(ca)2) potassium channels inhibit magnesium-dependent activity of the kinase-coupled cation channel TRPM7.
Chubanov V, Mederos y Schnitzler M, Meißner M, Schäfer S, Abstiens K, Hofmann T, Gudermann T. Chubanov V, et al. Br J Pharmacol. 2012 Jun;166(4):1357-76. doi: 10.1111/j.1476-5381.2012.01855.x. Br J Pharmacol. 2012. PMID: 22242975 Free PMC article. - Depletion of plasma membrane-associated phosphoinositides mimics inhibition of TRPM7 channels by cytosolic Mg2+, spermine, and pH.
Zhelay T, Wieczerzak KB, Beesetty P, Alter GM, Matsushita M, Kozak JA. Zhelay T, et al. J Biol Chem. 2018 Nov 23;293(47):18151-18167. doi: 10.1074/jbc.RA118.004066. Epub 2018 Oct 10. J Biol Chem. 2018. PMID: 30305398 Free PMC article. - Phagocytic activity of splenic macrophages is enhanced and accompanied by cytosolic alkalinization in TRPM7 kinase-dead mice.
Beesetty P, Rockwood J, Kaitsuka T, Zhelay T, Hourani S, Matsushita M, Kozak JA. Beesetty P, et al. FEBS J. 2021 Jun;288(11):3585-3601. doi: 10.1111/febs.15683. Epub 2021 Jan 6. FEBS J. 2021. PMID: 33354894 Free PMC article. - TRPM7: a unique channel involved in magnesium homeostasis.
Paravicini TM, Chubanov V, Gudermann T. Paravicini TM, et al. Int J Biochem Cell Biol. 2012 Aug;44(8):1381-4. doi: 10.1016/j.biocel.2012.05.010. Epub 2012 May 24. Int J Biochem Cell Biol. 2012. PMID: 22634382 Review. - The channel-kinase TRPM7, revealing the untold story of Mg(2+) in cellular signaling.
Schmitz C, Brandao K, Perraud AL. Schmitz C, et al. Magnes Res. 2014 Jan-Mar;27(1):9-15. doi: 10.1684/mrh.2014.0357. Magnes Res. 2014. PMID: 24752033 Review.
Cited by
- Natural and Synthetic Modulators of the TRPM7 Channel.
Chubanov V, Schäfer S, Ferioli S, Gudermann T. Chubanov V, et al. Cells. 2014 Nov 27;3(4):1089-101. doi: 10.3390/cells3041089. Cells. 2014. PMID: 25437439 Free PMC article. Review. - Defects in TRPM7 channel function deregulate thrombopoiesis through altered cellular Mg(2+) homeostasis and cytoskeletal architecture.
Stritt S, Nurden P, Favier R, Favier M, Ferioli S, Gotru SK, van Eeuwijk JM, Schulze H, Nurden AT, Lambert MP, Turro E, Burger-Stritt S, Matsushita M, Mittermeier L, Ballerini P, Zierler S, Laffan MA, Chubanov V, Gudermann T, Nieswandt B, Braun A. Stritt S, et al. Nat Commun. 2016 Mar 29;7:11097. doi: 10.1038/ncomms11097. Nat Commun. 2016. PMID: 27020697 Free PMC article. - Epidermal growth factor signaling through transient receptor potential melastatin 7 cation channel regulates vascular smooth muscle cell function.
Zou ZG, Rios FJ, Neves KB, Alves-Lopes R, Ling J, Baillie GS, Gao X, Fuller W, Camargo LL, Gudermann T, Chubanov V, Montezano AC, Touyz RM. Zou ZG, et al. Clin Sci (Lond). 2020 Aug 14;134(15):2019-2035. doi: 10.1042/CS20200827. Clin Sci (Lond). 2020. PMID: 32706027 Free PMC article. - TRPM6 and TRPM7 differentially contribute to the relief of heteromeric TRPM6/7 channels from inhibition by cytosolic Mg2+ and Mg·ATP.
Ferioli S, Zierler S, Zaißerer J, Schredelseker J, Gudermann T, Chubanov V. Ferioli S, et al. Sci Rep. 2017 Aug 18;7(1):8806. doi: 10.1038/s41598-017-08144-1. Sci Rep. 2017. PMID: 28821869 Free PMC article. - The zinc-binding motif of TRPM7 acts as an oxidative stress sensor to regulate its channel activity.
Inoue H, Murayama T, Kobayashi T, Konishi M, Yokoyama U. Inoue H, et al. J Gen Physiol. 2021 Jun 7;153(6):e202012708. doi: 10.1085/jgp.202012708. Epub 2021 May 17. J Gen Physiol. 2021. PMID: 33999118 Free PMC article.
References
- Nadler M. J. et al. LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature 411, 590–595 (2001). - PubMed
- Schlingmann K. P. et al. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat. Genet. 31, 166–170 (2002). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous