Translational control in synaptic plasticity and cognitive dysfunction - PubMed (original) (raw)
Review
Translational control in synaptic plasticity and cognitive dysfunction
Shelly A Buffington et al. Annu Rev Neurosci. 2014.
Abstract
Activity-dependent changes in the strength of synaptic connections are fundamental to the formation and maintenance of memory. The mechanisms underlying persistent changes in synaptic strength in the hippocampus, specifically long-term potentiation and depression, depend on new protein synthesis. Such changes are thought to be orchestrated by engaging the signaling pathways that regulate mRNA translation in neurons. In this review, we discuss the key regulatory pathways that govern translational control in response to synaptic activity and the mRNA populations that are specifically targeted by these pathways. The critical contribution of regulatory control over new protein synthesis to proper cognitive function is underscored by human disorders associated with either silencing or mutation of genes encoding proteins that directly regulate translation. In light of these clinical implications, we also consider the therapeutic potential of targeting dysregulated translational control to treat cognitive disorders of synaptic dysfunction.
Keywords: autism; eIF2α; local protein synthesis; mTOR; memory; neurodegeneration.
Figures
Figure 1
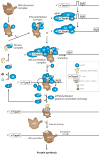
Translation initiation in neurons. Translation intitiation, often the rate-limiting step in protein synthesis, involves multiple fundamental reactions. These include formation of the 43S preinitiation complex, ribosomal scanning along the mRNA, AUG initiation codon recognition, and subunit joining to form the 80S ribosomal complex. Following 80S formation, translation elongation factors are recruited to elongate the forming polypeptide chain. Once the stop codon is reached, the elongation factors are disengaged and release of the newly synthesized protein is orchestrated through the action of translation termination factors.
Figure 2
The key signaling pathways that regulate activity-dependent translation initiation in neurons. (a) Synaptic activity triggers dephosphorylation of initiation factor eIF2α by either activation of eIF2α-specific phosphatase complexes or inhibition of the eIF2α kinases. Dephosphorylation of eIF2α is both sufficient and necessary to induce L-LTP and LTM formation. In glutamatergic neurons, phosphorylation of eIF2α promotes translation of ATF4, a CREB repressor. PKR-mediated phosphorylation of eIF2α in GABAergic neurons negatively regulates translation of the inflammatory cytokine IFN-γ, thus promoting GABA release and maintaining low network rhythmicity in the absence of significant stimulatory inputs. (b) Cap-dependent translation is mediated through mTORC1-dependent phosphorylation of its primary downstream effectors, the 4E-BPs and S6Ks. mTORC1 activity is driven by excitatory synaptic inputs, in addition to other cellular signals, that engage the PI3K/Akt signaling pathway. (c) Calcium influx following NMDAR activation triggers degradation of Paip2 by calcium-activated calpains, thus releasing PABP to bind eIF4G. The eIF4G-PABP complex then contributes to mRNA circularization by directly bridging the elongated 3′ poly(A) tail to the eIF4F complex at the 5′ cap. Cap-dependent translation is initiated upon subsequent recruitment of the 40S and 60S ribosomal complexes. Abbreviations: GADD34, growth arrest and DNA damage-inducible protein; CReP, constitutive reverter of eIF2α phosphorylation; CREB, cAMP response element-binding protein; PI3K, phosphatidylinositide 3-kinase; PDK1, phosphoinositide-dependent kinase-1.
Similar articles
- Translational regulatory mechanisms in synaptic plasticity and memory storage.
Costa-Mattioli M, Sonenberg N, Richter JD. Costa-Mattioli M, et al. Prog Mol Biol Transl Sci. 2009;90:293-311. doi: 10.1016/S1877-1173(09)90008-4. Epub 2009 Oct 27. Prog Mol Biol Transl Sci. 2009. PMID: 20374745 Review. - Translational regulatory mechanisms in persistent forms of synaptic plasticity.
Kelleher RJ 3rd, Govindarajan A, Tonegawa S. Kelleher RJ 3rd, et al. Neuron. 2004 Sep 30;44(1):59-73. doi: 10.1016/j.neuron.2004.09.013. Neuron. 2004. PMID: 15450160 Review. - Translational control of long-term synaptic plasticity and memory storage by eIF2alpha.
Costa-Mattioli M, Sonenberg N. Costa-Mattioli M, et al. Crit Rev Neurobiol. 2006;18(1-2):187-95. doi: 10.1615/critrevneurobiol.v18.i1-2.190. Crit Rev Neurobiol. 2006. PMID: 17725521 Review. - The Arc of cognition: Signaling cascades regulating Arc and implications for cognitive function and disease.
Epstein I, Finkbeiner S. Epstein I, et al. Semin Cell Dev Biol. 2018 May;77:63-72. doi: 10.1016/j.semcdb.2017.09.023. Semin Cell Dev Biol. 2018. PMID: 29559111 Free PMC article. Review. - Compartmentalized PDE4A5 Signaling Impairs Hippocampal Synaptic Plasticity and Long-Term Memory.
Havekes R, Park AJ, Tolentino RE, Bruinenberg VM, Tudor JC, Lee Y, Hansen RT, Guercio LA, Linton E, Neves-Zaph SR, Meerlo P, Baillie GS, Houslay MD, Abel T. Havekes R, et al. J Neurosci. 2016 Aug 24;36(34):8936-46. doi: 10.1523/JNEUROSCI.0248-16.2016. J Neurosci. 2016. PMID: 27559174 Free PMC article.
Cited by
- A unique binding mode of the eukaryotic translation initiation factor 4E for guiding the design of novel peptide inhibitors.
Di Marino D, D'Annessa I, Tancredi H, Bagni C, Gallicchio E. Di Marino D, et al. Protein Sci. 2015 Sep;24(9):1370-82. doi: 10.1002/pro.2708. Protein Sci. 2015. PMID: 26013047 Free PMC article. - The effects of proteasomal inhibition on synaptic proteostasis.
Hakim V, Cohen LD, Zuchman R, Ziv T, Ziv NE. Hakim V, et al. EMBO J. 2016 Oct 17;35(20):2238-2262. doi: 10.15252/embj.201593594. Epub 2016 Sep 9. EMBO J. 2016. PMID: 27613546 Free PMC article. - Capsosiphon fulvescens Glycoproteins Enhance Probiotics-Induced Cognitive Improvement in Aged Rats.
Oh JH, Nam TJ, Choi YH. Oh JH, et al. Nutrients. 2020 Mar 20;12(3):837. doi: 10.3390/nu12030837. Nutrients. 2020. PMID: 32245093 Free PMC article. - Dysregulation of Mammalian Target of Rapamycin Signaling in Mouse Models of Autism.
Huber KM, Klann E, Costa-Mattioli M, Zukin RS. Huber KM, et al. J Neurosci. 2015 Oct 14;35(41):13836-42. doi: 10.1523/JNEUROSCI.2656-15.2015. J Neurosci. 2015. PMID: 26468183 Free PMC article. Review. - Reduced protein synthesis in schizophrenia patient-derived olfactory cells.
English JA, Fan Y, Föcking M, Lopez LM, Hryniewiecka M, Wynne K, Dicker P, Matigian N, Cagney G, Mackay-Sim A, Cotter DR. English JA, et al. Transl Psychiatry. 2015 Oct 20;5(10):e663. doi: 10.1038/tp.2015.119. Transl Psychiatry. 2015. PMID: 26485547 Free PMC article.
References
- Banko JL, Merhav M, Stern E, Sonenberg N, Rosenblum K, Klann E. Behavioral alterations in mice lacking the translation repressor 4E-BP2. Neurobiol Learn Mem. 2007;87:248–56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous