Inhibition of autophagy, lysosome and VCP function impairs stress granule assembly - PubMed (original) (raw)
Inhibition of autophagy, lysosome and VCP function impairs stress granule assembly
S J Seguin et al. Cell Death Differ. 2014 Dec.
Abstract
Stress granules (SGs) are mRNA-protein aggregates induced during stress, which accumulate in many neurodegenerative diseases. Previously, the autophagy-lysosome pathway and valosin-containing protein (VCP), key players of the protein quality control (PQC), were shown to regulate SG degradation. This is consistent with the idea that PQC may survey and/or assist SG dynamics. However, despite these observations, it is currently unknown whether the PQC actively participates in SG assembly. Here, we describe that inhibition of autophagy, lysosomes and VCP causes defective SG formation after induction. Silencing the VCP co-factors UFD1L and PLAA, which degrade defective ribosomal products (DRIPs) and 60S ribosomes, also impaired SG assembly. Intriguingly, DRIPs and 60S, which are released from disassembling polysomes and are normally excluded from SGs, were significantly retained within SGs in cells with impaired autophagy, lysosome or VCP function. Our results suggest that deregulated autophagy, lysosomal or VCP activities, which occur in several neurodegenerative (VCP-associated) diseases, may alter SG morphology and composition.
Figures
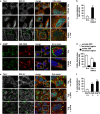
Figure 1
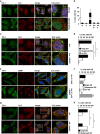
Lysosomotropic agents severely impair SG formation. HeLa cells treated for 3 h with MG132 alone or with ammonium chloride (NH4Cl; a, b) or chloroquine (CLQ; b) were fixed and labeled with anti-TIA-1, LC3 and DAPI. (b) Percentage of cells with TIA-1-positive SGs is shown (M=MG132; N=NH4Cl; C=CLQ). Error bar, S.E.M. ***P<0.001 compared with MG132. HeLa cells were treated for 45 min with 0.5 mM (c, d) or 0.1 mM (e, f) arsenite (Ars.); where indicated, cells were pretreated for 2 h 15 min with ammonium chloride (NH4Cl). Cells were fixed and labeled with anti-TIA-1, LC3 (c) or G3BP (e) and DAPI. (d, f) Percentage of cells with TIA-1-positive SGs (large or dispersed) and no SGs is shown. Error bar, S.E.M. (e) *P<0.05; **P<0.01; (f) ***P<0.001; **P<0.01 compared with Ars. 0.1 mM. (g, h) Hela cells pretreated or not with ammonium chloride (NH4Cl) for 2 h 15 min were subjected to heat shock (HS) at 43.5 °C for 45 min, fixed and labeled with anti-TIA-1, LC3 and DAPI. (h) Quantitation of data in g. Error bar, S.E.M. **P<0.01. (a, c, e, g) 2.5 × magnification of the selected area. See also Supplementary Figures S1, S2 and S3
Figure 2
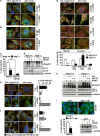
Atg5 and Atg16 null cells show impaired SG formation. (a–d) Atg5+/+ or Atg5−/− MEFs were left untreated or treated for 3 h with MG132 and/or ammonium chloride (NH4Cl) and either fixed and labeled with anti-TIA-1, LC3 and DAPI (a–c) or processed for western blot (d). (c) Quantitation of data in a and b. Error bar, S.E.M. **P<0.01 compared with MG132 in Atg5+/+. (e, f) Atg5+/+ and Atg5−/− MEFs were treated for 45 min with arsenite (Ars.); where indicated, MEFs were pretreated for 2 h 15 min with 20 mM ammonium chloride (NH4Cl). Cells were fixed and labeled with anti-TIA-1, LC3 and DAPI. Quantitation of data is shown. Error bar, S.E.M. ***P<0.001; *P<0.05 compared with Ars.; ##P<0.01 Ars-treated Atg5−/− compared with Atg5+/+. (g) Atg16+/+ and Atg16−/− MEFs were treated for 3 h with MG132 or for 45 min with Ars., fixed and labeled with anti-TIA-1, LC3 and DAPI. Quantitation of data is shown. Error bar, S.E.M. ***P<0.001; **P<0.01. (h) Atg16+/+ and Atg16−/− MEFs were treated as described in a; where indicated cells were pretreated for 2 h 15 min with ammonium chloride (NH4Cl), prior to lysis and western blot. (i) m5-7 cells were grown for 7 days without (−) or with (+) tetracycline (500 ng/ml), prior to addition of ammonium chloride (NH4Cl) or MG132 for 3 h. Cells were processed for western blot or fixed and labeled with anti-TIA-1 and DAPI. Quantitation of data is shown. Error bar, S.E.M. ***P<0.001 compared with MG132 condition. (h, i) * likely corresponds to LC3 T. (a, b, e–g) 2.5 × magnification of the selected area
Figure 3
Depletion and inhibition of VCP severely impair SG formation. (a–c) HeLa cells lipofected with control (ctrl) or VCP siRNA for 72 h were left untreated (data not shown) or treated for 3 h with MG132 or 45 min with arsenite (Ars.) Cells were lysed, processed for western blot (a) or fixed and labeled with anti-TIA-1, LC3 and DAPI (b). (c) Quantitation of data in b. Error bar, S.E.M. **P<0.01; ***P<0.001. (d) HeLa cells not treated or treated for 3 h with ML240 or EerI were lysed and processed for ubiquitin, PARP (c.f.: cleaved-fragment), Hsp70, LC3 and α-tubulin western blot. (e) Cells were treated for 3 h with MG132 or for 45 min with Ars. alone or in combination with ML240 or EerI; cells were fixed and labeled with anti-TIA-1, G3BP and DAPI. Quantitation of data is shown. Error bar, S.E.M. For MG132 co-treatments: ***P<0.001 compared with MG132 alone; for Ars. co-treatments: ***P<0.001; **P<0.01; *P<0.05 compared with Ars. alone. (f) Schematic model of the known role of VCP in targeting DRIPs and large ribosome subunits (60S) to degradation. Polysomes are a cluster of ribosomes that synthesize nascent chains from mRNAs. Upon stress, including MG132 or arsenite treatments, polysomes disassemble and their components follow different fates. mRNAs together with initiation factors and the small (40S) ribosome subunit are sequestered into stress granules (SGs). DRIPs are ubiquitinated and routed to degradation. VCP bound to 60S modulates the degradation of DRIPs. Co-factors such as UFD1L and PLAA assist VCP in client targeting to both proteasome and autophagy for clearance. In parallel, 60S are also released but are excluded from SGs and can become damaged under stress conditions (e.g., oxidation due to arsenite). 60S are targeted to lysosome for degradation, a process called ribophagy. VCP and PLAA are also required in yeast for ribophagy. (b, e, f) 2.5 × magnification of the selected area. See also Supplementary Figure S4
Figure 4
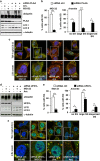
Depletion of the VCP co-factors PLAA and UFD1L affects SG formation. HeLa cells lipofected with control (ctrl), PLAA (a–c) or UFD1L siRNA (d–f) for 72 h were left untreated (data not shown) or treated for 3 h with MG132 or 45 min with arsenite (Ars). Cells were lysed and processed for western blot (a, d), or fixed and labeled with anti-TIA-1, LC3 and DAPI (c, f). (b, e) Quantitation of data in c and f. Error bar, S.E.M. ***P<0.001; **P<0.01; *P<0.05. (c, f) 2.5 × magnification of the selected area. See also Supplementary Figure S4
Figure 5
Inhibition of proteasome and lysosome or silencing of VCP and co-factors lead to the accumulation of OP-puro-labeled DRIPs adjacent to or within SGs. (a–c) HeLa cells were co-treated for 45 min with OP-puro and arsenite (Ars.); where indicated, cells were pretreated with bortezomib (Bort.) overnight and/or ammonium chloride (NH4Cl) for 2 h 15 min. Cells were fixed and labeled with Alexa594–Azide and anti-TIA-1. (b) The number of OP-puro puncta per cell is shown. Error bar, S.E.M. ***P<0.001 compared with Ars. (c) The percentage of OP-puro puncta adjacent to/colocalizing with SGs is shown. Error bar, S.E.M. ***P<0.001 compared with Ars. (d) HeLa cells lipofected for 72 h with control, VCP, PLAA or UFD1L siRNA were treated for 45 min with OP-puro and Ars., fixed and stained with Alexa594–Azide, anti-TIA-1 and DAPI. Arrowheads indicate OP-puro-labeled DRIPs colocalizing with TIA-1-positive SGs. The percentage of OP-puro puncta adjacent to/colocalizing with SGs is shown. Error bar, S.E.M. ***P<0.001 compared with cells transfected with control siRNA. (a, d) 2.5 × magnification of the selected area. See also Supplementary Figure S5
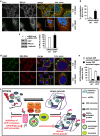
Figure 6
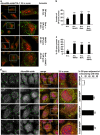
Chemical inhibition of lysosomes or genetic perturbation of autophagy leads to formation of SGs that contain RPL19. (a, b) HeLa cells were treated for 45 min with arsenite (Ars.) alone or following ammonium chloride (NH4Cl) pre-treatment. Cells were fixed and labeled with anti-TIA-1, RPL19 and DAPI. (b) Quantitation of colocalization of RPL19 and TIA-1 is shown. Error bar, S.E.M. **P<0.01. (c, d) Cells treated as described in a were fixed, subjected to in situ hybridization with a 28S rRNA probe coupled to Alexa594 and labeled with anti-G3BP and DAPI. (d) Quantitation of the % of TIA-1-positive SGs located outside or inside the 28S rRNA perinuclear-enriched region is shown. Error bar, S.E.M. *P<0.05. (e, f) Atg16+/+, Atg5−/− and Atg16−/− MEFs were treated for 45 min with Ars. and labeled with anti-TIA-1, RPL19 and DAPI. (f) Quantitation of colocalization of RPL19 and TIA-1 is shown. Error bar, S.E.M. **P<0.01. (a–c) 2.5 × magnification of the selected area. See also Supplementary Figure S6
Figure 7
RPL19 and 28S rRNA partly colocalize with arsenite-induced SGs in VCP-depleted cells. HeLa cells lipofected for 72 h with control or VCP siRNA were treated for 45 min with arsenite (Ars.), fixed and labeled with anti-TIA-1, RPL19 and DAPI (a, b) or subjected to western blot (c) or to in situ hybridization with a 28S rRNA probe, followed by co-staining with anti-G3BP and DAPI (d, e). (b) Quantitation of colocalization of RPL19 and TIA-1 is shown. Error bar, S.E.M. **P<0.01. (c) Quantitation of RPL19 protein levels is shown. Error bar, S.E.M. *P<0.05. (e) Quantitation of the % of TIA-1-positive SGs located outside or inside the 28S rRNA perinuclear-enriched region is shown. Error bar, S.E.M. *P<0.05. (f) Schematic model of the proposed interplay between VCP, autophagy and SGs. Upon stress polysomes disassemble releasing DRIPs and ribosome subunits (40S and 60S), while mRNA-binding proteins containing prion-like domains (e.g., TIA-1) trigger the sequestration of bound mRNAs into SGs. SGs also contain, besides initiation factors, 40S and the autophagy protein LC3. Instead, DRIPs and 60S are excluded from assembled SGs. DRIPs and ubiquitinated unfolded proteins are targeted to degradation by proteasome and autolysosomes with the assistance of VCP and its co-factors UFD1L and PLAA. Also damaged 60S can be targeted to degradation by proteasome and/or lysosome, a process that also involves VCP and co-factors, while undamaged 60S can be recycled (not shown). Inhibition of autophagy or lysosomes, as well as depletion of VCP (and the co-factors PLAA or UFD1L) leads to the accumulation of DRIPs and 60S within the foci where SGs are assembling. Under such conditions, the SG response is decreased (in term of SG size and number) and SGs contain the non-canonical components DRIPs and 60S. (a, d) 2.5 × magnification of the selected area
Similar articles
- BAG3 and BAG6 differentially affect the dynamics of stress granules by targeting distinct subsets of defective polypeptides released from ribosomes.
Mediani L, Galli V, Carrà AD, Bigi I, Vinet J, Ganassi M, Antoniani F, Tiago T, Cimino M, Mateju D, Cereda C, Pansarasa O, Alberti S, Mandrioli J, Carra S. Mediani L, et al. Cell Stress Chaperones. 2020 Nov;25(6):1045-1058. doi: 10.1007/s12192-020-01141-w. Epub 2020 Jul 21. Cell Stress Chaperones. 2020. PMID: 32696179 Free PMC article. - Pathogenic variants of Valosin-containing protein induce lysosomal damage and transcriptional activation of autophagy regulators in neuronal cells.
Ferrari V, Cristofani R, Cicardi ME, Tedesco B, Crippa V, Chierichetti M, Casarotto E, Cozzi M, Mina F, Galbiati M, Piccolella M, Carra S, Vaccari T, Nalbandian A, Kimonis V, Fortuna TR, Pandey UB, Gagliani MC, Cortese K, Rusmini P, Poletti A. Ferrari V, et al. Neuropathol Appl Neurobiol. 2022 Aug;48(5):e12818. doi: 10.1111/nan.12818. Epub 2022 May 15. Neuropathol Appl Neurobiol. 2022. PMID: 35501124 Free PMC article. - Eukaryotic stress granules are cleared by autophagy and Cdc48/VCP function.
Buchan JR, Kolaitis RM, Taylor JP, Parker R. Buchan JR, et al. Cell. 2013 Jun 20;153(7):1461-74. doi: 10.1016/j.cell.2013.05.037. Cell. 2013. PMID: 23791177 Free PMC article. - Granulostasis: Protein Quality Control of RNP Granules.
Alberti S, Mateju D, Mediani L, Carra S. Alberti S, et al. Front Mol Neurosci. 2017 Mar 27;10:84. doi: 10.3389/fnmol.2017.00084. eCollection 2017. Front Mol Neurosci. 2017. PMID: 28396624 Free PMC article. Review. - Stress granules at the intersection of autophagy and ALS.
Monahan Z, Shewmaker F, Pandey UB. Monahan Z, et al. Brain Res. 2016 Oct 15;1649(Pt B):189-200. doi: 10.1016/j.brainres.2016.05.022. Epub 2016 May 13. Brain Res. 2016. PMID: 27181519 Free PMC article. Review.
Cited by
- TDRD3 functions as a selective autophagy receptor with dual roles in autophagy and modulation of stress granule stability.
Deater M, Lloyd RE. Deater M, et al. bioRxiv [Preprint]. 2024 Sep 22:2024.09.22.614367. doi: 10.1101/2024.09.22.614367. bioRxiv. 2024. PMID: 39345463 Free PMC article. Preprint. - ALS' Perfect Storm: _C9orf72_-Associated Toxic Dipeptide Repeats as Potential Multipotent Disruptors of Protein Homeostasis.
Smeele PH, Cesare G, Vaccari T. Smeele PH, et al. Cells. 2024 Jan 17;13(2):178. doi: 10.3390/cells13020178. Cells. 2024. PMID: 38247869 Free PMC article. Review. - Valosin containing protein (VCP): initiator, modifier, and potential drug target for neurodegenerative diseases.
Chu S, Xie X, Payan C, Stochaj U. Chu S, et al. Mol Neurodegener. 2023 Aug 7;18(1):52. doi: 10.1186/s13024-023-00639-y. Mol Neurodegener. 2023. PMID: 37545006 Free PMC article. Review. - Ubiquitin Modulates Liquid-Liquid Phase Separation of UBQLN2 via Disruption of Multivalent Interactions.
Dao TP, Kolaitis RM, Kim HJ, O'Donovan K, Martyniak B, Colicino E, Hehnly H, Taylor JP, Castañeda CA. Dao TP, et al. Mol Cell. 2018 Mar 15;69(6):965-978.e6. doi: 10.1016/j.molcel.2018.02.004. Epub 2018 Mar 8. Mol Cell. 2018. PMID: 29526694 Free PMC article. - An aberrant phase transition of stress granules triggered by misfolded protein and prevented by chaperone function.
Mateju D, Franzmann TM, Patel A, Kopach A, Boczek EE, Maharana S, Lee HO, Carra S, Hyman AA, Alberti S. Mateju D, et al. EMBO J. 2017 Jun 14;36(12):1669-1687. doi: 10.15252/embj.201695957. Epub 2017 Apr 4. EMBO J. 2017. PMID: 28377462 Free PMC article.
References
- Bukau B, Weissman J, Horwich A. Molecular chaperones and protein quality control. Cell. 2006;125:443–451. - PubMed
- Kim YE, Hipp MS, Bracher A, Hayer-Hartl M, Hartl FU. Molecular chaperone functions in protein folding and proteostasis. Annu Rev Biochem. 2013;82:323–355. - PubMed
- Schubert U, Anton LC, Gibbs J, Norbury CC, Yewdell JW, Bennink JR. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature. 2000;404:770–774. - PubMed
- Anderson P, Kedersha N. Stress granules. Curr Biol. 2009;19:R397–R398. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous