Amyloidogenic peptide oligomer accumulation in autophagy-deficient β cells induces diabetes - PubMed (original) (raw)
. 2014 Aug;124(8):3311-24.
doi: 10.1172/JCI69625. Epub 2014 Jul 18.
Hwanju Cheon, Yeon Taek Jeong, Wenying Quan, Kook Hwan Kim, Jae Min Cho, Yu-Mi Lim, Seung Hoon Oh, Sang-Man Jin, Jae Hyeon Kim, Moon-Kyu Lee, Sunshin Kim, Masaaki Komatsu, Sang-Wook Kang, Myung-Shik Lee
- PMID: 25036705
- PMCID: PMC4109549
- DOI: 10.1172/JCI69625
Amyloidogenic peptide oligomer accumulation in autophagy-deficient β cells induces diabetes
Jinyoung Kim et al. J Clin Invest. 2014 Aug.
Abstract
Islet amyloid accumulation is a hallmark of human type 2 diabetes (T2D). In contrast to human islet amyloid polypeptide (hIAPP), murine islet amyloid polypeptide (mIAPP) does not exhibit amyloidogenic propensity. Because autophagy is important in the clearance of amyloid-like proteins, we studied transgenic mice with β cell-specific expression of hIAPP to evaluate the contribution of autophagy in T2D-associated accumulation of hIAPP. In mice with β cell-specific expression of hIAPP, a deficiency in autophagy resulted in development of overt diabetes, which was not observed in mice expressing hIAPP alone or lacking autophagy alone. Furthermore, lack of autophagy in hIAPP-expressing animals resulted in hIAPP oligomer and amyloid accumulation in pancreatic islets, leading to increased death and decreased mass of β cells. Expression of hIAPP in purified monkey islet cells or a murine β cell line resulted in pro-hIAPP dimer formation, while dimer formation was absent or reduced dramatically in cells expressing either nonamyloidogenic mIAPP or nonfibrillar mutant hIAPP. In autophagy-deficient cells, accumulation of pro-hIAPP dimers increased markedly, and pro-hIAPP trimers were detected in the detergent-insoluble fraction. Enhancement of autophagy improved the metabolic profile of hIAPP-expressing mice fed a high-fat diet. These results suggest that autophagy promotes clearance of amyloidogenic hIAPP, autophagy deficiency exacerbates pathogenesis of human T2D, and autophagy enhancers have therapeutic potential for islet amyloid accumulation-associated human T2D.
Figures
Figure 7. Proposed model of pro-hIAPP dimer and trimer formation.
The site of pro-hIAPP dimerization would be the membrane-rich fraction rather than the soluble fraction, since, after binding of pro-hIAPP to the membrane fraction, the local concentration of pro-hIAPP will increase and the encounter between molecules will be restricted to 2 dimensions (54). Some of the pro-hIAPP dimer will be translocated to the soluble fraction, while other pro-hIAPP dimer would proceed to form pro-hIAPP trimer, probably in the membrane-rich fraction. Although the exact molecular structure of the pro-hIAPP trimer is unknown, a recently proposed parallel stacking model with a U-bend conformation of β-strand–loop–β-strand (47, 62) is shown.
Figure 6. Effects of autophagy enhancer on the glucose profile of hIAPP+ mice.
(A) Primary mouse islet cells were treated with 100 mM trehalose for 24 hours with or without 100 nM bafilomycin pretreatment to block lysosomal steps of autophagy. Cell lysates were subjected to Western blot analysis using anti-LC3 or anti-p62 Ab. (B) Trehalose (2 g/kg) was administered i.p. to 12-week-old GFP-LC3+ mice on HFD for 2 weeks, and frozen pancreas sections were prepared for confocal microscopy to examine LC3 puncta. Scale bar: 50 μm. (C) Trehalose (2 g/kg) was administered i.p. to 16- to 20-week-old hIAPP+ mice on HFD, and nonfasting blood glucose levels were monitored (n = 5). (D) IPGTT was performed after 4 weeks of trehalose administration to hIAPP+ mice on HFD (n = 5). (E) The insulinogenic index was calculated after 4 weeks of trehalose administration to hIAPP+ mice on HFD (n = 3). (F) After immunofluorescent staining using A11 Ab, the percentage of A11-stained cells among total DAPI+ islet cells was determined by confocal microscopy. Representative pictures are shown. Scale bar: 50 μm. (G) After FSB staining, mean fluorescence intensity per islet area was determined using the NIS-Elements AR 3.0 software (Nikon). Representative pictures are shown. Scale bar: 100 μm. *P < 0.05, **P < 0.01, ***P < 0.001; #P < 0.05, ##P < 0.01, ###P < 0.001. (*, comparison between hIAPP+/HFD + trehalose and hIAPP+/HFD + PBS groups; #, comparison between hIAPP+/HFD + PBS and hIAPP–/HFD + PBS groups in C and D.)
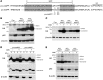
Figure 5. Pro-hIAPP dimer and trimer.
(A) Amino acid sequences of pro-IAPP used in this study. The highly conserved region between hIAPP and mIAPP is indicated in gray boxes. Cleavage sites of proprotein convertase 2 (left) and 1/3 (right) are indicated with “X”s. Asterisks indicate mutation sites in which the original amino acids were changed to prolines in pro-hIAPPmt. (B) INS-1 cells were transfected with indicated constructs for 24 hours and incubated with or without 5 mM 3-MA for additional 24 hours. Cell lysates were subjected to Western blot analysis using anti-HA Ab. Equal protein loading and autophagy inhibition were confirmed by β-actin and p62 levels, respectively. (C) After transfection of INS-1 cells with indicated constructs and treatment with 3-MA as in B, cell lysates were prepared and subjected to Western blot analysis. (D) INS-1 cells were transfected and treated as in B. The detergent-soluble (S) and detergent-insoluble (P) pellet fractions were subjected to Western blot analysis using anti-HA Ab. Equal protein loading was confirmed by β-actin level. Differential migration of monomeric pro-IAPPs between detergent-soluble and -insoluble fractions was due to the difference in the detergents used. exp., exposure. (E) Primary monkey islet cells were transfected with the indicated constructs for 24 hours and incubated with or without 100 nM bafilomycin for an additional 24 hours. Cell lysates were subjected to Western blot analysis using anti-HA Ab. Equal protein loading and autophagy inhibition were confirmed by β-actin and p62 level, respectively.
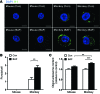
Figure 4. Accumulation of I11-stained IAPP oligomer in primary monkey islet cells.
(A) Primary monkey islet cells or mouse islet cells were incubated with 100 nM bafilomycin (BAF) that efficiently blocks autophagy of primary cells or solvent alone (Con) for 24 hours. Confocal microscopy was performed after immunofluorescent staining using I11 Ab. Scale bar: 5 μm. (B) The number of I11-stained puncta was counted. (C) After the same incubation of primary monkey and mouse islet cells with or without bafilomycin, apoptosis was determined by measuring oligonucleosomal content in cell extract using a Cell Death Detection ELISA Kit (Roche). **P < 0.01, ***P < 0.001.
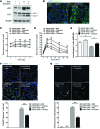
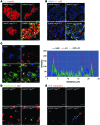
Figure 3. In vivo accumulation of hIAPP oligomer and amyloid.
(A) Confocal microscopy after immunofluorescent staining of pancreas sections from 12-week-old mice using anti-insulin and anti-hIAPP oligomer Abs (I11). Arrowheads indicate colocalization of I11 and insulin immunostaining. Scale bar: 50 μm. (B) Confocal microscopy after immunofluorescent staining using anti-p62 and I11 Ab and subsequent DAPI staining to identify nuclei. The arrowhead indicates rare colocalization of I11 and p62 immunostaining. Scale bar: 50 μm. (C) Confocal microscopy after immunofluorescent staining of hIAPP+/+GFP-LC3+ mouse islets using A11 Ab. Line tracing performed along the white dashed line in the merged picture to visualize colocalization of A11-stained hIAPP oligomer and GFP-LC3+ autophagosomes shows overlapping of hIAPP fluorescence with GFP fluorescence. Scale bar: 5 μm. (D and E) Confocal microscopy after immunofluorescent staining of pancreas sections from 12-week-old mice using (D) anti-p62 Ab and thioflavin-S staining or (E) using anti-ubiquitin Ab and FSB staining, as described in the Methods. Arrowheads indicate amyloid stained with thioflavin-S (green) or FSB (blue). Scale bar: 5 μm.
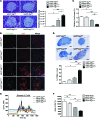
Figure 2. β Cells in hIAPP+Atg7_Δβ_cell mice.
(A) H&E staining of pancreas sections and the number of cells detached from surrounding tissues or cells per islet area in 12- to 15-week-old mice (n = 3~6). Scale bar: 100 μm. (B) Confocal microscopy after immunofluorescent staining of pancreas sections from 12- to 15-week-old mice using anti-p62 and -ubiquitin Abs. Scale bar: 100 μm. (C) Relative β cell mass in 12- to 15-week-old mice measured by insulin IHC and the point counting method (n = 3 each). (D) The percentage of apoptotic TUNEL+ β cells among total β cells in islets of 12- to 15-week-old mice (n = 4~5). Representative TUNEL staining is shown. Arrowheads indicate TUNEL+ β cells. Scale bar: 100 μm. (E) Glucose-induced Ca2+ transients in isolated islets (n = 4 each). Ca2+ transients were measured in Tyrode solution containing 3.0 or 16.7 mM glucose, as described in the Methods. (F) Δ[Ca2+]c area was defined as the total area of [Ca2+]c above basal [Ca2+]c. *P < 0.05, **P < 0.01, ***P < 0.001. ns, not significant.
Figure 1. Development of diabetes in hIAPP+Atg7_Δβ_cell mice.
(A) RT-PCR analysis of hIAPP and Atg7 expression in primary islets. The residual expression of Atg7 in islets of hIAPP–Atg7_Δβ_cell or hIAPP+Atg7_Δβ_cell mice is probably due to non–β cells in primary islets. (B) Nonfasting blood glucose levels in male and female mice (n = 33~46 for male and 30~51 for female mice between 8 and 20 week of age; n = 10~16 for male and 10~19 for female mice before 8 weeks of age). (C) Body weight of male mice. Body weight of female mice was also not different between groups (data not shown). The numbers of mice in C are the same as those in B. (D) IPGTT after overnight fasting in 12- to 15-week-old male (n = 5~7) and female mice (n = 12~23). (E) Serum insulin levels in fasted 12- to 15-week-old male mice determined by ELISA (n = 10 each). (F) The insulinogenic index was calculated from 12- to 15-week-old male mice (n = 5~7). *P < 0.05, **P < 0.01, ***P < 0.001; #P < 0.05, ##P < 0.01, ###P < 0.001. (*, comparison with hIAPP+Atg7fl/fl mice; #, comparison with hIAPP–Atg7_Δβ_cell mice in B and D.)
Comment in
- Islet amyloid and type 2 diabetes: overproduction or inadequate clearance and detoxification?
Gupta D, Leahy JL. Gupta D, et al. J Clin Invest. 2014 Aug;124(8):3292-4. doi: 10.1172/JCI77506. Epub 2014 Jul 18. J Clin Invest. 2014. PMID: 25036704 Free PMC article. - Diabetes: Protective role of autophagy in pancreatic β cells.
Osório J. Osório J. Nat Rev Endocrinol. 2014 Oct;10(10):575. doi: 10.1038/nrendo.2014.144. Epub 2014 Aug 12. Nat Rev Endocrinol. 2014. PMID: 25112233 No abstract available.
Similar articles
- UCHL1 deficiency exacerbates human islet amyloid polypeptide toxicity in β-cells: evidence of interplay between the ubiquitin/proteasome system and autophagy.
Costes S, Gurlo T, Rivera JF, Butler PC. Costes S, et al. Autophagy. 2014 Jun;10(6):1004-14. doi: 10.4161/auto.28478. Autophagy. 2014. PMID: 24879150 Free PMC article. - An autophagy enhancer ameliorates diabetes of human IAPP-transgenic mice through clearance of amyloidogenic oligomer.
Kim J, Park K, Kim MJ, Lim H, Kim KH, Kim SW, Lee ES, Kim HH, Kim SJ, Hur KY, Kim JH, Ahn JH, Yoon KH, Kim JW, Lee MS. Kim J, et al. Nat Commun. 2021 Jan 8;12(1):183. doi: 10.1038/s41467-020-20454-z. Nat Commun. 2021. PMID: 33420039 Free PMC article. - Human IAPP-induced pancreatic β cell toxicity and its regulation by autophagy.
Shigihara N, Fukunaka A, Hara A, Komiya K, Honda A, Uchida T, Abe H, Toyofuku Y, Tamaki M, Ogihara T, Miyatsuka T, Hiddinga HJ, Sakagashira S, Koike M, Uchiyama Y, Yoshimori T, Eberhardt NL, Fujitani Y, Watada H. Shigihara N, et al. J Clin Invest. 2014 Aug;124(8):3634-44. doi: 10.1172/JCI69866. Epub 2014 Jul 18. J Clin Invest. 2014. PMID: 25036706 Free PMC article. - Role of Autophagy in the Pathogenesis of Diabetes and Therapeutic Potential of Autophagy Modulators in the Treatment of Diabetes and Metabolic Syndrome.
Oh SJ, Lee MS. Oh SJ, et al. J Korean Med Sci. 2022 Sep 26;37(37):e276. doi: 10.3346/jkms.2022.37.e276. J Korean Med Sci. 2022. PMID: 36163475 Free PMC article. Review. - The β-cell assassin: IAPP cytotoxicity.
Raleigh D, Zhang X, Hastoy B, Clark A. Raleigh D, et al. J Mol Endocrinol. 2017 Oct;59(3):R121-R140. doi: 10.1530/JME-17-0105. Epub 2017 Aug 15. J Mol Endocrinol. 2017. PMID: 28811318 Review.
Cited by
- Glibenclamide-Induced Autophagy Inhibits Its Insulin Secretion-Improving Function in β Cells.
Zhou J, Kang X, Luo Y, Yuan Y, Wu Y, Wang M, Liu D. Zhou J, et al. Int J Endocrinol. 2019 Aug 15;2019:1265175. doi: 10.1155/2019/1265175. eCollection 2019. Int J Endocrinol. 2019. PMID: 31511772 Free PMC article. - Hallmarks of Aging: An Autophagic Perspective.
Barbosa MC, Grosso RA, Fader CM. Barbosa MC, et al. Front Endocrinol (Lausanne). 2019 Jan 9;9:790. doi: 10.3389/fendo.2018.00790. eCollection 2018. Front Endocrinol (Lausanne). 2019. PMID: 30687233 Free PMC article. Review. - Immune regulation of islet homeostasis and adaptation.
Guo J, Fu W. Guo J, et al. J Mol Cell Biol. 2020 Oct 1;12(10):764-774. doi: 10.1093/jmcb/mjaa009. J Mol Cell Biol. 2020. PMID: 32236479 Free PMC article. Review. - Urolithins: Diet-Derived Bioavailable Metabolites to Tackle Diabetes.
Raimundo AF, Ferreira S, Tomás-Barberán FA, Santos CN, Menezes R. Raimundo AF, et al. Nutrients. 2021 Nov 27;13(12):4285. doi: 10.3390/nu13124285. Nutrients. 2021. PMID: 34959837 Free PMC article. Review. - Adapt, Recycle, and Move on: Proteostasis and Trafficking Mechanisms in Melanoma.
Demirsoy S, Martin S, Maes H, Agostinis P. Demirsoy S, et al. Front Oncol. 2016 Nov 15;6:240. doi: 10.3389/fonc.2016.00240. eCollection 2016. Front Oncol. 2016. PMID: 27896217 Free PMC article. Review.
References
- Kahn SE. The relative contribution of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia. 2003;46(1):3–19. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases