Human IAPP-induced pancreatic β cell toxicity and its regulation by autophagy - PubMed (original) (raw)
. 2014 Aug;124(8):3634-44.
doi: 10.1172/JCI69866. Epub 2014 Jul 18.
Ayako Fukunaka, Akemi Hara, Koji Komiya, Akira Honda, Toyoyoshi Uchida, Hiroko Abe, Yukiko Toyofuku, Motoyuki Tamaki, Takeshi Ogihara, Takeshi Miyatsuka, Henry J Hiddinga, Setsuya Sakagashira, Masato Koike, Yasuo Uchiyama, Tamotsu Yoshimori, Norman L Eberhardt, Yoshio Fujitani, Hirotaka Watada
- PMID: 25036706
- PMCID: PMC4109539
- DOI: 10.1172/JCI69866
Human IAPP-induced pancreatic β cell toxicity and its regulation by autophagy
Nayumi Shigihara et al. J Clin Invest. 2014 Aug.
Abstract
Pancreatic islets in patients with type 2 diabetes mellitus (T2DM) are characterized by loss of β cells and formation of amyloid deposits derived from islet amyloid polypeptide (IAPP). Here we demonstrated that treatment of INS-1 cells with human IAPP (hIAPP) enhances cell death, inhibits cytoproliferation, and increases autophagosome formation. Furthermore, inhibition of autophagy increased the vulnerability of β cells to the cytotoxic effects of hIAPP. Based on these in vitro findings, we examined the pathogenic role of hIAPP and its relation to autophagy in hIAPP-knockin mice. In animals fed a standard diet, hIAPP had no toxic effects on β cell function; however, hIAPP-knockin mice did not exhibit a high-fat-diet-induced compensatory increase in β cell mass, which was due to limited β cell proliferation and enhanced β cell apoptosis. Importantly, expression of hIAPP in mice with a β cell-specific autophagy defect resulted in substantial deterioration of glucose tolerance and dispersed cytoplasmic expression of p62-associated toxic oligomers, which were otherwise sequestrated within p62-positive inclusions. Together, our results indicate that increased insulin resistance in combination with reduced autophagy may enhance the toxic potential of hIAPP and enhance β cell dysfunction and progression of T2DM.
Figures
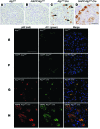
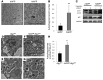
Figure 7. hIAPP inhibits formation of cytoplasmic inclusions immunopositive for p62 in Atg7fl/fl:Cre islets.
(A–D) Representative section of the pancreas of 22-week-old high-fat diet–fed Atg7fl/fl (A), hIAPP:Atg7fl/fl (B), Atg7fl/fl:Cre (C), and hIAPP:Atg7fl/fl:Cre (D) mice, immunohistochemically stained for p62. The p62-positive signal accumulated as inclusions (arrows) within islet cells of Atg7fl/fl:Cre mice, but were dispersed throughout the cytoplasm in hIAPP:Atg7fl/fl:Cre mice. Scale bar: 200 μm. (E–H) Confocal images of pancreas sections from 22-week-old high-fat diet–fed Atg7fl/fl (E), hIAPP:Atg7fl/fl (F), Atg7fl/fl:Cre (G), and hIAPP:Atg7fl/fl:Cre (H) mice stained for p62, A11, and cell nuclei (blue). Staining of toxic oligomer was intense in Atg7fl/fl:Cre and hIAPP:Atg7fl/fl:Cre islets, but faint in Atg7fl/fl and hIAPP:Atg7fl/fl islets. p62 was largely colocalized with A11-positive staining in Atg7fl/fl:Cre and hIAPP:Atg7fl/fl:Cre islets. Scale bar: 50 μm.
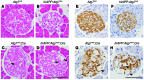
Figure 6. Islet morphology.
Shown is the morphology of representative islets from Atg7fl/fl (A and E), hIAPP:Atg7fl/fl (B and F), hIAPP:Atg7fl/fl (C and G), and hIAPP:Atg7fl/fl:Cre (D and H) mice fed high-fat diet from 8 to 20 weeks of age. Arrowheads indicate degenerative “balloon-like” β cells. (A–D) H&E staining. (E–H) Insulin staining. Scale bars: 50 μm.
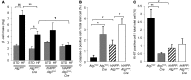
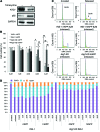
Figure 5. Effect of hIAPP knockin and autophagy deficiency on β cell mass.
(A) β cell mass of Atg7fl/fl, Atg7fl/fl:Cre, hIAPP:Atg7fl/fl, and hIAPP:Atg7fl/fl:Cre mice fed standard (STD) or high-fat (HF) diet. (B and C) Number of cleaved caspase-3–positive (B) and Ki-67–positive (C) intraislet cells in high-fat diet–fed mice of each genotype. In A–C, 5 mice per genotype were prepared for morphometric analysis. Data are mean ± SEM. §P < 0.05, §§P < 0.01, standard vs. high-fat; *P < 0.05, **P < 0.01, Atg7fl/fl vs. Atg7fl/fl:Cre; †P < 0.05, hIAPP:Atg7fl/fl vs. hIAPP:Atg7fl/fl:Cre; #P < 0.05, Atg7fl/fl vs. hIAPP:Atg7fl/fl; ¶P < 0.05, Atg7fl/fl:Cre vs. hIAPP:Atg7fl/fl:Cre.
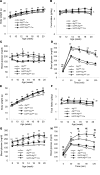
Figure 4. Physiological parameters after adjustment for body weight.
Atg7fl/fl (n = 9), Atg7fl/fl:Cre (n = 11), hIAPP:Atg7fl/fl (n = 10), and hIAPP:Atg7fl/fl:Cre (n = 11) mice were fed high-fat diet from 8 to 20 weeks of age as in Figure 3, E–H. Adjusted values for (A) body weight, (B) nonfasting blood glucose, and (C) glucose tolerance correspond to results in Figure 3, E, G, and H, respectively. Data are mean ± SEM. **P < 0.01, Atg7fl/fl vs. Atg7fl/fl:Cre; †P < 0.05, ††P < 0.01, hIAPP:Atg7fl/fl vs. hIAPP:Atg7fl/fl:Cre; #P < 0.05, Atg7fl/fl vs. hIAPP:Atg7fl/fl; ¶P < 0.05, Atg7fl/fl:Cre vs. hIAPP:Atg7fl/fl:Cre.
Figure 3. Physiological parameters.
Mice were fed standard diet (A–D) or high-fat diet (E–H) from 8 to 20 weeks of age. Shown are body weight (A and E), food intake (B and F), nonfasting blood glucose levels (C and G), and glucose tolerance (D and H) of Atg7fl/fl (n = 31 [standard], 49 [high-fat]), Atg7fl/fl:Cre (n = 38 [standard], 39 [high-fat]), hIAPP:Atg7fl/fl (n = 31 [standard], 32 [high-fat]), and hIAPP:Atg7fl/fl:Cre (n = 19 [standard], 27 [high-fat]) mice. Data are mean ± SEM. *P < 0.05, **P < 0.01, Atg7fl/fl vs. Atg7fl/fl:Cre; †P < 0.05, ††P < 0.01, hIAPP:Atg7fl/fl vs. hIAPP:Atg7fl/fl:Cre; #P < 0.05, ##P < 0.01, Atg7fl/fl vs. hIAPP:Atg7fl/fl; ¶P < 0.05, ¶¶P < 0.01, Atg7fl/fl:Cre vs. hIAPP:Atg7fl/fl:Cre.
Figure 2. Effects of hIAPP/rIAPP and autophagy deficiency on cell cycle and viability of INS-1 cells.
(A) Association of reduced ATG7 with accumulation of p62 in Atg7-KD INS-1 cells with tetracycline supplementation. GAPDH served as loading control. (B) INS-1 and Atg7-KD INS-1 cells were cultured in the presence of 0–4 μM rIAPP or hIAPP peptide for 48 hours. The number of cells not stained with Trypan Blue was counted; cell number is presented as percentage of control (0 μM IAPP). *P < 0.05, **_P_ < 0.01, INS-1 rIAPP vs. INS-1 hIAPP; ††_P_ < 0.01, INS-1 rIAPP vs. Atg7-KD rIAPP; ##_P_ < 0.01, Atg7-KD rIAPP vs. Atg7-KD hIAPP; ¶_P_ < 0.05, INS-1 hIAPP vs. Atg7-KD hIAPP. (**C** and **D**) FACS histograms (**C**) and stacked bar graphs (**D**) of INS-1 cells grown under conditions of growth arrest (induced by exposure to 2.8 mMol/l glucose and serum withdrawal for >24 hours) and after release from arrest (by 22.2 mMol/l glucose, 200 nMol/l insulin, hIAPP/rIAPP, and serum withdrawal medium for 24 hours).
Figure 1. Induction of autophagy by hIAPP.
(A) EM examination showed autophagosome formation in INS-1 cells treated for 12 hours with rIAPP or hIAPP peptide. Arrowheads indicate autophagosomes. Scale bar: 2 μm. (B) Number of autophagosomes per cell. **P < 0.01, rIAPP vs. hIAPP. (C) Protein levels of LC3, p62, and GAPDH, assessed by immunoblotting. To visualize accumulation of LC3-II, the lysosomal inhibitors pepstatin A and E64d were added to the culture medium for an additional 6 hours. (D–G) EM examination showed autophagosome formation in β cells of 22-week-old Atg7fl/fl (D and E) and hIAPP:Atg7fl/fl (F and G) mice fed high-fat diet from 8 to 20 weeks of age. (E and G) Magnified images of autophagosomes (arrows) in D and F, respectively. Scale bars: 2 μm. (H) Autophagosome counts. #P < 0.05, Atg7fl/fl vs. hIAPP:Atg7fl/fl.
Comment in
- Islet amyloid and type 2 diabetes: overproduction or inadequate clearance and detoxification?
Gupta D, Leahy JL. Gupta D, et al. J Clin Invest. 2014 Aug;124(8):3292-4. doi: 10.1172/JCI77506. Epub 2014 Jul 18. J Clin Invest. 2014. PMID: 25036704 Free PMC article. - Diabetes: Protective role of autophagy in pancreatic β cells.
Osório J. Osório J. Nat Rev Endocrinol. 2014 Oct;10(10):575. doi: 10.1038/nrendo.2014.144. Epub 2014 Aug 12. Nat Rev Endocrinol. 2014. PMID: 25112233 No abstract available.
Similar articles
- Amyloidogenic peptide oligomer accumulation in autophagy-deficient β cells induces diabetes.
Kim J, Cheon H, Jeong YT, Quan W, Kim KH, Cho JM, Lim YM, Oh SH, Jin SM, Kim JH, Lee MK, Kim S, Komatsu M, Kang SW, Lee MS. Kim J, et al. J Clin Invest. 2014 Aug;124(8):3311-24. doi: 10.1172/JCI69625. Epub 2014 Jul 18. J Clin Invest. 2014. PMID: 25036705 Free PMC article. - UCHL1 deficiency exacerbates human islet amyloid polypeptide toxicity in β-cells: evidence of interplay between the ubiquitin/proteasome system and autophagy.
Costes S, Gurlo T, Rivera JF, Butler PC. Costes S, et al. Autophagy. 2014 Jun;10(6):1004-14. doi: 10.4161/auto.28478. Autophagy. 2014. PMID: 24879150 Free PMC article. - Autophagy defends pancreatic β cells from human islet amyloid polypeptide-induced toxicity.
Rivera JF, Costes S, Gurlo T, Glabe CG, Butler PC. Rivera JF, et al. J Clin Invest. 2014 Aug;124(8):3489-500. doi: 10.1172/JCI71981. Epub 2014 Jul 18. J Clin Invest. 2014. PMID: 25036708 Free PMC article. - The β-cell assassin: IAPP cytotoxicity.
Raleigh D, Zhang X, Hastoy B, Clark A. Raleigh D, et al. J Mol Endocrinol. 2017 Oct;59(3):R121-R140. doi: 10.1530/JME-17-0105. Epub 2017 Aug 15. J Mol Endocrinol. 2017. PMID: 28811318 Review. - Human IAPP amyloidogenic properties and pancreatic β-cell death.
Fernández MS. Fernández MS. Cell Calcium. 2014 Nov;56(5):416-27. doi: 10.1016/j.ceca.2014.08.011. Epub 2014 Aug 27. Cell Calcium. 2014. PMID: 25224501 Review.
Cited by
- Current Status of Autophagy Enhancers in Metabolic Disorders and Other Diseases.
Park K, Lee MS. Park K, et al. Front Cell Dev Biol. 2022 Feb 14;10:811701. doi: 10.3389/fcell.2022.811701. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35237600 Free PMC article. Review. - Urolithin B: Two-way attack on IAPP proteotoxicity with implications for diabetes.
Raimundo AF, Ferreira S, Pobre V, Lopes-da-Silva M, Brito JA, Dos Santos DJVA, Saraiva N, Dos Santos CN, Menezes R. Raimundo AF, et al. Front Endocrinol (Lausanne). 2022 Dec 15;13:1008418. doi: 10.3389/fendo.2022.1008418. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36589826 Free PMC article. - Cellular Autophagy in α Cells Plays a Role in the Maintenance of Islet Architecture.
Himuro M, Miyatsuka T, Suzuki L, Miura M, Katahira T, Goto H, Nishida Y, Sasaki S, Koike M, Shiota C, Gittes GK, Fujitani Y, Watada H. Himuro M, et al. J Endocr Soc. 2019 Aug 8;3(11):1979-1992. doi: 10.1210/js.2019-00075. eCollection 2019 Nov 1. J Endocr Soc. 2019. PMID: 31620668 Free PMC article. - Diabetes Drug Discovery: hIAPP1-37 Polymorphic Amyloid Structures as Novel Therapeutic Targets.
Fernández-Gómez I, Sablón-Carrazana M, Bencomo-Martínez A, Domínguez G, Lara-Martínez R, Altamirano-Bustamante NF, Jiménez-García LF, Pasten-Hidalgo K, Castillo-Rodríguez RA, Altamirano P, Marrero SR, Revilla-Monsalve C, Valdés-Sosa P, Salamanca-Gómez F, Garrido-Magaña E, Rodríguez-Tanty C, Altamirano-Bustamante MM. Fernández-Gómez I, et al. Molecules. 2018 Mar 19;23(3):686. doi: 10.3390/molecules23030686. Molecules. 2018. PMID: 29562662 Free PMC article. - Intermittent fasting preserves beta-cell mass in obesity-induced diabetes via the autophagy-lysosome pathway.
Liu H, Javaheri A, Godar RJ, Murphy J, Ma X, Rohatgi N, Mahadevan J, Hyrc K, Saftig P, Marshall C, McDaniel ML, Remedi MS, Razani B, Urano F, Diwan A. Liu H, et al. Autophagy. 2017;13(11):1952-1968. doi: 10.1080/15548627.2017.1368596. Epub 2017 Nov 25. Autophagy. 2017. PMID: 28853981 Free PMC article.
References
- Kahn SE. The relative contributions of insulin resistance and β-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia. 2003;46(1):3–19. - PubMed
- Kloppel G, Lohr M, Habich K, Oberholzer M, Heitz PU. Islet pathology and the pathogenesis of type 1 and type 2 diabetes mellitus revisited. Surv Synth Pathol Res. 1985;4(2):110–125. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases