Dynamic heterogeneity and DNA methylation in embryonic stem cells - PubMed (original) (raw)
Dynamic heterogeneity and DNA methylation in embryonic stem cells
Zakary S Singer et al. Mol Cell. 2014.
Abstract
Cell populations can be strikingly heterogeneous, composed of multiple cellular states, each exhibiting stochastic noise in its gene expression. A major challenge is to disentangle these two types of variability and to understand the dynamic processes and mechanisms that control them. Embryonic stem cells (ESCs) provide an ideal model system to address this issue because they exhibit heterogeneous and dynamic expression of functionally important regulatory factors. We analyzed gene expression in individual ESCs using single-molecule RNA-FISH and quantitative time-lapse movies. These data discriminated stochastic switching between two coherent (correlated) gene expression states and burst-like transcriptional noise. We further showed that the "2i" signaling pathway inhibitors modulate both types of variation. Finally, we found that DNA methylation plays a key role in maintaining these metastable states. Together, these results show how ESC gene expression states and dynamics arise from a combination of intrinsic noise, coherent cellular states, and epigenetic regulation.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
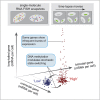
Graphical abstract
Figure 1
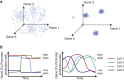
Different Types of Gene Expression Heterogeneity (A) Intrinsic noise in gene expression can lead to uncorrelated variation (left), while the coexistence of distinct cellular states can produce correlated variability in gene expression (right). Both panels depict schematic static “snapshots” of gene expression. (B) Dynamically, gene expression levels could vary infrequently and abruptly (left) or more frequently and gradually (right) both within and between cellular states (schematic).
Figure 2
smFISH Reveals Gene Expression Heterogeneity and Correlation (A) Top: coefficients of variation (CV, mean ± SEM) for ESC-associated regulators and housekeeping genes. Bottom: Distributions (violin plots) normalized by maximum expression level reveal qualitatively distinct gene expression distributions. Genes are sorted by increasing CV. (B) Smoothed histograms for mRNA distributions overlaid with NB fits. Solid lines show individual NB distributions. Dashed gray lines show their sum (for bimodal genes). ∗ denotes 95th percentile for Prdm14. P value: χ2 goodness of fit test. (C) Pairwise relationships between genes, analyzed by smFISH (r, Pearson correlation coefficient; p value by 2D K-S test (see Experimental Procedures and Figures S2A and S2B). (D) Heat maps show examples of four-dimensional data sets.
Figure 3
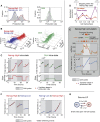
The Two Rex1 States Are Differentially Methylated (A) smFISH measurements show that Rex1 bimodality is correlated with Tet1 and anticorrelated with Dnmt3b expression. (B) Locus-specific bisulfite sequencing of the Dazl promoter. Methylation levels shown are in the _Rex1_-high (top), _Rex1_-low (middle), and _Rex1_-low-to-high reverting (bottom) populations. (C) Global levels of 5mC measured by quantitative ELISA in the _Rex1_-high, -low, and -low-to-high reverting cells. Data shown are mean ± SD from two independent experiments. ∗, p < 0.05; ∗∗, p < 0.01; by two-sample t test. (D) Histogram of promoter methylation shows bimodality in the _Rex1_-high (top) and -low (bottom) states, as quantified by RRBS. (E) Scatter plot of promoter methylation between _Rex1_-high and -low states. Each point is the methylation fraction of a single gene promoter, color-coded by the number of CpGs in that promoter. Divergence from the diagonal implies differential methylation between states. Inset: Single CpGs in the promoter of the specific gene labeled, color coded by distance from TSS; see Figure S3C for additional genes.
Figure 4
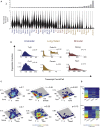
Movies Reveal Transcriptional Bursting and State-Switching Dynamics in Individual Cells (A) Distribution of Nanog and Oct4 production rates from representative movies in serum + LIF, and Gaussian fits to the components. Production rates were extracted from a total of 376 and 103 tracked cell cycles for Nanog and Oct4, respectively. (B) Production rate distributions of individual cell lineage trees, each consisting of closely related cells descending from a single cell. Lineage trees are color-coded by the state they spend the majority of time in. (C) Example single lineage traces exhibiting step-like changes in production rates within a state. (D) Cell cycle phase distribution of steps within the _Nanog_-high state. Step occurrences are normalized by the frequencies of each cell cycle phase observed in the tracked data. (E) Representative trace showing apparent steps from simulations under the bursty transcription model, using parameters estimated from mRNA distribution for the _Nanog_-high state (see Supplemental Information; see Figure S4E for simulation of Oct4 dynamics). (F) Example traces of individual cells switching between _Nanog_-low and _Nanog_-high states. (G) Empirical transition rates (mean ± SD) between the two Nanog states (NHi, _Nanog_-high; NLo, _Nanog_-low).
Figure 5
2i and DNA Methylation Modulate Bursty Transcription and State-Switching Dynamics (A) Comparison of mRNA distributions and CV between cells grown in serum + LIF and 2i + serum + LIF. Top: For each gene, the CV in serum + LIF is plotted on the left, and the CV for 2i + serum + LIF is plotted on the right. Dnmt3b in 2i + serum + LIF is represented in gray to reflect its marginal case of poor quality of fit in both bimodal and long-tailed models. Bottom: The left half of each violin represents the mRNA distribution in serum + LIF, while the right represents 2i + serum + LIF. Each gene’s distributions are normalized by a value corresponding to the larger 95th percentile between the two treatments. (B) Distribution of Nanog production rates from movies in 2i + serum + LIF. (C) Empirical transition rates between the two Nanog states in the presence of 2i (NLo, _Nanog_-low; NSH, _Nanog_-SH). (D) Mixing time in each condition is estimated from autocorrelation, A(τ), of production rate ranks shown in Figure S5D, right panels. Red, _Nanog_-high in serum + LIF; purple, _Nanog_-SH in 2i + serum + LIF. Error bars: standard deviation, bootstrap method. (E) Comparison of transcriptional heterogeneity between Dnmt TKO (black line) and the parental line (blue bars) as measured by smFISH for Rex1, Nanog, Esrrb, and SDHA. Note that for Rex1/Nanog/Essrb, there are fewer “off” cells in the leftmost bins for the TKO than WT. (F) _Rex1_-dGFP distribution as measured by flow cytometry grown in serum + LIF with 5-aza or DMSO (carrier control). Time points were taken after 2, 4, and 6 days. (G) Cells were grown in 2i + serum + LIF and subsequently replated into serum + LIF with 5-aza or DMSO (carrier control). Time points were taken after 2, 4, and 6 days. GFP levels were measured by flow cytometry.
Similar articles
- Integrative single-cell omics analyses reveal epigenetic heterogeneity in mouse embryonic stem cells.
Luo Y, He J, Xu X, Sun MA, Wu X, Lu X, Xie H. Luo Y, et al. PLoS Comput Biol. 2018 Mar 21;14(3):e1006034. doi: 10.1371/journal.pcbi.1006034. eCollection 2018 Mar. PLoS Comput Biol. 2018. PMID: 29561833 Free PMC article. - Dynamic heterogeneity of DNA methylation and hydroxymethylation in embryonic stem cell populations captured by single-cell 3D high-content analysis.
Tajbakhsh J, Stefanovski D, Tang G, Wawrowsky K, Liu N, Fair JH. Tajbakhsh J, et al. Exp Cell Res. 2015 Mar 15;332(2):190-201. doi: 10.1016/j.yexcr.2015.02.004. Epub 2015 Feb 17. Exp Cell Res. 2015. PMID: 25700729 Free PMC article. - Stochastic NANOG fluctuations allow mouse embryonic stem cells to explore pluripotency.
Abranches E, Guedes AM, Moravec M, Maamar H, Svoboda P, Raj A, Henrique D. Abranches E, et al. Development. 2014 Jul;141(14):2770-9. doi: 10.1242/dev.108910. Development. 2014. PMID: 25005472 Free PMC article. - Transcription regulation and chromatin structure in the pluripotent ground state.
Marks H, Stunnenberg HG. Marks H, et al. Biochim Biophys Acta. 2014 Mar;1839(3):129-37. doi: 10.1016/j.bbagrm.2013.09.005. Epub 2013 Oct 2. Biochim Biophys Acta. 2014. PMID: 24096207 Review. - Genomic studies to explore self-renewal and differentiation properties of embryonic stem cells.
Zhan M. Zhan M. Front Biosci. 2008 Jan 1;13:276-83. doi: 10.2741/2678. Front Biosci. 2008. PMID: 17981546 Review.
Cited by
- Nanog, Oct4 and Tet1 interplay in establishing pluripotency.
Olariu V, Lövkvist C, Sneppen K. Olariu V, et al. Sci Rep. 2016 May 5;6:25438. doi: 10.1038/srep25438. Sci Rep. 2016. PMID: 27146218 Free PMC article. - What's Luck Got to Do with It: Single Cells, Multiple Fates, and Biological Nondeterminism.
Symmons O, Raj A. Symmons O, et al. Mol Cell. 2016 Jun 2;62(5):788-802. doi: 10.1016/j.molcel.2016.05.023. Mol Cell. 2016. PMID: 27259209 Free PMC article. Review. - SCRABBLE: single-cell RNA-seq imputation constrained by bulk RNA-seq data.
Peng T, Zhu Q, Yin P, Tan K. Peng T, et al. Genome Biol. 2019 May 6;20(1):88. doi: 10.1186/s13059-019-1681-8. Genome Biol. 2019. PMID: 31060596 Free PMC article. - Lineages of embryonic stem cells show non-Markovian state transitions.
Udomlumleart T, Hu S, Garg S. Udomlumleart T, et al. iScience. 2021 Jul 17;24(8):102879. doi: 10.1016/j.isci.2021.102879. eCollection 2021 Aug 20. iScience. 2021. PMID: 34401663 Free PMC article. - A narrative review of tumor heterogeneity and challenges to tumor drug therapy.
Zhu L, Jiang M, Wang H, Sun H, Zhu J, Zhao W, Fang Q, Yu J, Chen P, Wu S, Zheng Z, He Y. Zhu L, et al. Ann Transl Med. 2021 Aug;9(16):1351. doi: 10.21037/atm-21-1948. Ann Transl Med. 2021. PMID: 34532488 Free PMC article. Review.
References
- Blake W.J., KAErn M., Cantor C.R., Collins J.J. Noise in eukaryotic gene expression. Nature. 2003;422:633–637. - PubMed
- Borgel J., Guibert S., Li Y., Chiba H., Schübeler D., Sasaki H., Forné T., Weber M. Targets and dynamics of promoter DNA methylation during early mouse development. Nat. Genet. 2010;42:1093–1100. - PubMed
- Cai L., Friedman N., Xie X.S. Stochastic protein expression in individual cells at the single molecule level. Nature. 2006;440:358–362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01HD075605A/HD/NICHD NIH HHS/United States
- P50 GM068763/GM/NIGMS NIH HHS/United States
- R01 GM086793/GM/NIGMS NIH HHS/United States
- 092096/Wellcome Trust/United Kingdom
- R01GM086793A/GM/NIGMS NIH HHS/United States
- R01 HD075605/HD/NICHD NIH HHS/United States
- P50GM068763/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases