SGLT2 inhibitor lowers serum uric acid through alteration of uric acid transport activity in renal tubule by increased glycosuria - PubMed (original) (raw)
Randomized Controlled Trial
. 2014 Oct;35(7):391-404.
doi: 10.1002/bdd.1909. Epub 2014 Aug 6.
Affiliations
- PMID: 25044127
- PMCID: PMC4223977
- DOI: 10.1002/bdd.1909
Free PMC article
Randomized Controlled Trial
SGLT2 inhibitor lowers serum uric acid through alteration of uric acid transport activity in renal tubule by increased glycosuria
Yukihiro Chino et al. Biopharm Drug Dispos. 2014 Oct.
Free PMC article
Abstract
Sodium glucose cotransporter 2 (SGLT2) inhibitors have been reported to lower the serum uric acid (SUA) level. To elucidate the mechanism responsible for this reduction, SUA and the urinary excretion rate of uric acid (UE(UA)) were analysed after the oral administration of luseogliflozin, a SGLT2 inhibitor, to healthy subjects. After dosing, SUA decreased, and a negative correlation was observed between the SUA level and the UE(UA), suggesting that SUA decreased as a result of the increase in the UE(UA). The increase in UE(UA) was correlated with an increase in urinary D-glucose excretion, but not with the plasma luseogliflozin concentration. Additionally, in vitro transport experiments showed that luseogliflozin had no direct effect on the transporters involved in renal UA reabsorption. To explain that the increase in UE(UA) is likely due to glycosuria, the study focused on the facilitative glucose transporter 9 isoform 2 (GLUT9ΔN, SLC2A9b), which is expressed at the apical membrane of the kidney tubular cells and transports both UA and D-glucose. It was observed that the efflux of [(14) C]UA in Xenopus oocytes expressing the GLUT9 isoform 2 was trans-stimulated by 10 mm D-glucose, a high concentration of glucose that existed under SGLT2 inhibition. On the other hand, the uptake of [(14) C]UA by oocytes was cis-inhibited by 100 mm D-glucose, a concentration assumed to exist in collecting ducts. In conclusion, it was demonstrated that the UE(UA) could potentially be increased by luseogliflozin-induced glycosuria, with alterations of UA transport activity because of urinary glucose.
Keywords: GLUT9; SGLT2 inhibitor; sodium glucose cotransporter; uric acid; urinary glucose excretion.
© 2014 The Authors. Biopharmaceutics & Drug Disposition. Published by John Wiley & Sons Ltd.
Figures
Figure 1
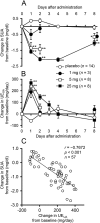
Effect of luseogliflozin on the serum uric acid (SUA) level. Changes in the SUA level from the baseline after a single dose (A, n = 3–14) and after multiple doses (B, n = 8) are shown. Data are mean ± SEM. **p < 0.01 vs placebo (0 mg) (Dunnett’s test)
Figure 2
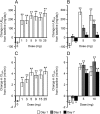
Effects of luseogliflozin on the urinary excretion rate (_UE_UA) and the renal clearance (_CL_UA) of uric acid. Changes in _UE_UA and _CL_UA from the baseline after a single dose (A and C, n = 3–14) and after multiple doses (B and D, n = 8) are shown. Data are mean ± SEM. **p < 0.01 vs placebo (0 mg) (Dunnett’s test)
Figure 3
Relationship between the serum uric acid (SUA) level and the urinary excretion rate of uric acid (_UE_UA) after a single dose. The daily changes in the SUA level (A) and _UE_UA (B) from the baseline in the 1, 5 and 25 mg dosing groups are shown. Data are mean ± SEM. *p < 0.05, **p < 0.01 vs placebo (Dunnett’s test, Student’s _t_-test or Aspin-Welch’s _t_-test). (C) Correlation between the changes in the SUA level and the _UE_UA on day 1 from the baseline values in the single dose study
Figure 4
Comparison of the urinary excretion rate of uric acid (_UE_UA), the plasma concentration of luseogliflozin and the urinary excretion rate of glucose (_UE_GL) in the single dose study. (A–C) Time course profiles of _UE_UA, the plasma concentration of luseogliflozin and _UE_GL. Data are mean ± SEM. (D) Relationship between _UE_UA and _UE_GL. (E) Relationship between _UE_UA and _AUC_0–24h of plasma luseogliflozin
Figure 5
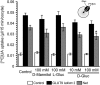
Effect of luseogliflozin on transporters involved in renal uric acid handling in humans. URAT1 (A) and OAT4 (C) were examined in gene-expressing HEK293 and S2 cells, respectively. GLUT9 isoform 1 (B), OAT10 (D) and SMCT1 (E) were examined in gene-expressing Xenopus oocytes. Data are mean ± SEM
Figure 6
_Trans_-stimulatory effect of
d
-glucose on uric acid transport by GLUT9 isoform 2. Effect of the injected amount of cRNA of GLUT9 isoform 2 into Xenopus oocytes on UA efflux (n = 7–8) determined at 5 min and (B) time-course of UA efflux from the oocytes injected with 0.25 ng of cRNA (closed circle) or water (open circle) (n = 4–8). (C) _Trans_-stimulatory effect of
d
-glucose (n = 5–10) and (D) _cis_-inhibitory effect of benzbromarone (Benz) (n = 7–10) on GLUT9 isoform 2-mediated [14C]UA efflux. (E) _Tran_s-stimulatory effect of
d
-glucose on GLUT9 isoform 2-mediated [14C]UA uptake (n = 14–17). The efflux and uptake of [14C]UA were evaluated using oocytes injected with 0.25 ng and 25 ng of cRNA, respectively. Data are mean ± SEM. *p < 0.05 vs 10 m
m l
-glucose (C, Dunnett’s test) or vs 10 m
m d
-glucose (D, Aspin-Welch’s _t_-test), **p < 0.01 vs 100 m
m l
-glucose (E, Aspin-Welch’s _t_-test)
Figure 7
_Cis_-inhibitory effect of
d
-glucose on GLUT9 isoform 2-mediated [14C]UA uptake in oocytes injected with 25 ng of cRNA (n = 8–12). Data are mean ± SEM. *p < 0.05 vs control (transport buffer) (Aspin-Welch’s _t_-test), Significant difference was not observed as a result of multiple comparison (Dunnett’s test) was used
Figure 8
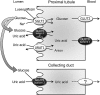
Proposed model for uricosuric effect of SGLT2 inhibitors by glycosuria-induced uric acid secretion via GLUT9 isoform 2 or any other functionally similar transporters at the proximal tubule and inhibition of uric acid uptake via GLUT9 isoform 2 at the collecting duct of renal tubule
Similar articles
- Factors Influencing Change in Serum Uric Acid After Administration of the Sodium-Glucose Cotransporter 2 Inhibitor Luseogliflozin in Patients With Type 2 Diabetes Mellitus.
Chino Y, Kuwabara M, Hisatome I. Chino Y, et al. J Clin Pharmacol. 2022 Mar;62(3):366-375. doi: 10.1002/jcph.1970. Epub 2021 Nov 19. J Clin Pharmacol. 2022. PMID: 34545949 Free PMC article. Clinical Trial. - SGLT2 inhibition and renal urate excretion: role of luminal glucose, GLUT9, and URAT1.
Novikov A, Fu Y, Huang W, Freeman B, Patel R, van Ginkel C, Koepsell H, Busslinger M, Onishi A, Nespoux J, Vallon V. Novikov A, et al. Am J Physiol Renal Physiol. 2019 Jan 1;316(1):F173-F185. doi: 10.1152/ajprenal.00462.2018. Epub 2018 Nov 14. Am J Physiol Renal Physiol. 2019. PMID: 30427222 Free PMC article. - Mechanism-Based Pharmacokinetic-Pharmacodynamic Modeling of Luseogliflozin, a Sodium Glucose Co-transporter 2 Inhibitor, in Japanese Patients with Type 2 Diabetes Mellitus.
Samukawa Y, Mutoh M, Chen S, Mizui N. Samukawa Y, et al. Biol Pharm Bull. 2017;40(8):1207-1218. doi: 10.1248/bpb.b16-00998. Biol Pharm Bull. 2017. PMID: 28769002 Clinical Trial. - Effects of Sodium Glucose Cotransporter-2 Inhibitors on Serum Uric Acid in Type 2 Diabetes Mellitus.
Ahmadieh H, Azar S. Ahmadieh H, et al. Diabetes Technol Ther. 2017 Sep;19(9):507-512. doi: 10.1089/dia.2017.0070. Epub 2017 Jul 27. Diabetes Technol Ther. 2017. PMID: 28749169 Review. - Luseogliflozin for the treatment of type 2 diabetes.
Seino Y. Seino Y. Expert Opin Pharmacother. 2014 Dec;15(18):2741-9. doi: 10.1517/14656566.2014.978290. Epub 2014 Oct 30. Expert Opin Pharmacother. 2014. PMID: 25359155 Review.
Cited by
- The Pathophysiological Basis of Diabetic Kidney Protection by Inhibition of SGLT2 and SGLT1.
Oe Y, Vallon V. Oe Y, et al. Kidney Dial. 2022 Jun;2(2):349-368. doi: 10.3390/kidneydial2020032. Epub 2022 Jun 18. Kidney Dial. 2022. PMID: 36380914 Free PMC article. - Decreased Associated Risk of Gout in Diabetes Patients with Uric Acid Urolithiasis.
Liu CJ, Wu JS, Huang HS. Liu CJ, et al. J Clin Med. 2019 Sep 25;8(10):1536. doi: 10.3390/jcm8101536. J Clin Med. 2019. PMID: 31557790 Free PMC article. - Management of diabolical diabetes mellitus and periodontitis nexus: Are we doing enough?
Gurav AN. Gurav AN. World J Diabetes. 2016 Feb 25;7(4):50-66. doi: 10.4239/wjd.v7.i4.50. World J Diabetes. 2016. PMID: 26962409 Free PMC article. Review. - Safe and pragmatic use of sodium-glucose co-transporter 2 inhibitors in type 2 diabetes mellitus: South Asian Federation of Endocrine Societies consensus statement.
Kalra S, Ghosh S, Aamir AH, Ahmed MT, Amin MF, Bajaj S, Baruah MP, Bulugahapitiya U, Das AK, Giri M, Gunatilake S, Mahar SA, Pathan MF, Qureshi NK, Raza SA, Sahay R, Shakya S, Shreshta D, Somasundaram N, Sumanatilleke M, Unnikrishnan AG, Wijesinghe AM. Kalra S, et al. Indian J Endocrinol Metab. 2017 Jan-Feb;21(1):210-230. doi: 10.4103/2230-8210.196029. Indian J Endocrinol Metab. 2017. PMID: 28217523 Free PMC article. Review. - Sodium-glucose Co-transporter 2 Inhibitors Reduce the Abdominal Visceral Fat Area and May Influence the Renal Function in Patients with Type 2 Diabetes.
Tosaki T, Kamiya H, Himeno T, Kato Y, Kondo M, Toyota K, Nishida T, Shiroma M, Tsubonaka K, Asai H, Moribe M, Nakaya Y, Nakamura J. Tosaki T, et al. Intern Med. 2017;56(6):597-604. doi: 10.2169/internalmedicine.56.7196. Epub 2017 Mar 17. Intern Med. 2017. PMID: 28321056 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources