Population of sensory neurons essential for asthmatic hyperreactivity of inflamed airways - PubMed (original) (raw)
Population of sensory neurons essential for asthmatic hyperreactivity of inflamed airways
Dimitri Tränkner et al. Proc Natl Acad Sci U S A. 2014.
Abstract
Asthma is a common debilitating inflammatory lung disease affecting over 200 million people worldwide. Here, we investigated neurogenic components involved in asthmatic-like attacks using the ovalbumin-sensitized murine model of the disease, and identified a specific population of neurons that are required for airway hyperreactivity. We show that ablating or genetically silencing these neurons abolished the hyperreactive broncho-constrictions, even in the presence of a fully developed lung inflammatory immune response. These neurons are found in the vagal ganglia and are characterized by the expression of the transient receptor potential vanilloid 1 (TRPV1) ion channel. However, the TRPV1 channel itself is not required for the asthmatic-like hyperreactive airway response. We also demonstrate that optogenetic stimulation of this population of TRP-expressing cells with channelrhodopsin dramatically exacerbates airway hyperreactivity of inflamed airways. Notably, these cells express the sphingosine-1-phosphate receptor 3 (S1PR3), and stimulation with a S1PR3 agonist efficiently induced broncho-constrictions, even in the absence of ovalbumin sensitization and inflammation. Our results show that the airway hyperreactivity phenotype can be physiologically dissociated from the immune component, and provide a platform for devising therapeutic approaches to asthma that target these pathways separately.
Keywords: airway inflammation; bronchospasms.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
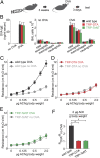
Genetic ablation or silencing of sensory neurons abolishes asthma-like airway hyperreactivity. (A) Schematic of the standard ovalbumin-sensitized mouse model for acute asthma (21). (B) The levels of ovalbumin (OVA)-specific IgE (Left) and the numbers of immune cells in broncho-alveolar lavage (BAL) (Center and Right) are similar in control, TRP-DTA, and TRP-TeNT animals after OVA-sensitization (n ≥ 6). (C) Airway resistance in response to acetylcholine stimulation (3, 22) in wild-type mice (n ≥10). (D) Airway responses in TRP-DTA mice (n ≥ 5), and (E) in TRP-TeNT mice (n ≥ 3). (F) Ovalbumin induces a dramatic change of airway responses in wild-type, but not in TRP-DTA and TRP-TeNT mice (both t test *P < 0.001). Data represent means ± SEM.
Fig. 2.
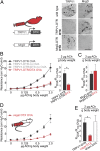
Vagal TRPV1-expressing sensory neurons mediate allergic airway hyperreactivity. (A) Selective ablation of sensory neurons is achieved by injecting TRPV1-DTR or MrgD-DTR mice with DTX. TRPV1-DTR mice treated with DTX lose TRPV1-cells, but retain MrgD-expressing cells. Conversely, DTX treated MrgD-DTR mice lose MrgD-expressing cells, but retain TRPV1-cells. (Scale bar: 100 μm.) (B) Airway resistance in response to acetylcholine in TRPV1-DTR control mice (n ≥ 3) and in animals challenged with DTX (TRPV1-DTR/DTX; n = 6). Ovalbumin changes airway responses in TRPV1-DTR but not in TRPV1-DTR/DTX mice (t test *P = 0.003). (C) DTX-treated MrgD-DTR mice exhibit normal ovalbumin induced airway hyperreactivity (n ≥ 3). (D) Airway resistance in response to acetylcholine in TRPV1-DTR mice challenged with direct vagal DTX injections (n = 3). The control responses shown as dashed lines (no DTX injection) are taken from B for comparison purposes. (E) Quantification of airway responses in vagal and sham DTX injections (i.e., DTX injections just outside the vagal ganglia, n = 4). Data represent means ± SEM; t test *P = 0.02.
Fig. 3.
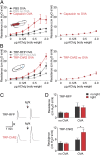
Activation of vagal TRPV1-cells exacerbates allergic airway hyperreactivity. (A) Airway resistance in response to acetylcholine in C57BL/6 mice 1 h after vagal injection of PBS (n ≥ 5) and 300 µM capsaicin (n ≥ 4). (B) Airway resistance in response to acetylcholine in TRP-RFP and TRP-ChR2 mice (n ≥ 5) after 1 h of vagal light stimulation. Note that stimulation of lung sensory neurons using optogenetics (or capsaicin, see A) has no effect on the baseline airway resistance in the absence of acetylcholine injections. (C) Recordings of airway resistance before and after light stimulation in an ovalbumin-sensitized TRP-RFP (Upper) and TRP-ChR2 (Lower) animal; small arrowhead indicates the time of acetylcholine injection (0.5 µg/g body weight). R = 3 cm H2O·s/mL (D) Quantification of ChR2 responses. Vagal light stimulation increases the airway response in ovalbumin-sensitized TRP-ChR2 (n = 7; t test *P = 0.04). In contrast, light has no effect on nonsensitized TRP-ChR2 or ovalbumin sensitized TRP-RFP control mice (n = 5). Data were obtained with 0.5 µg acetylcholine per gram body weight. Data represent means ± SEM.
Fig. 4.
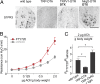
S1P receptor activation induces airway hyperreactivity. (A) ISH experiments demonstrate that vagal neurons expressing S1PR3 are ablated in TRP-DTA and DTX-treated TRPV1-DTR mice, but not in control DTX-treated MrgD-DTR mice. (Scale bar: 100 μm.) (B) The S1PR3 agonist FTY720 (see Materials and Methods for dose and route of treatment) induces strong airway hyperreactivity in the absence of ovalbumen sensitization (n ≥
4
; t test P < 0.001). (C) The effect of FTY720 on airway responses is abolished in animals without sensory neurons (TRP-DTA, n = 3; t test *P = 0.010) and enhanced in mice with optogenetically activated vagal sensory neurons (TRP-ChR2, n = 4; t test *P = 0.003). Data represent means ± SEM.
Similar articles
- The functional consequences of structural changes in the airways: implications for airway hyperresponsiveness in asthma.
Wang L, McParland BE, Paré PD. Wang L, et al. Chest. 2003 Mar;123(3 Suppl):356S-62S. Chest. 2003. PMID: 12628973 Review. No abstract available. - Pan-neurotrophin receptor p75 contributes to neuronal hyperreactivity and airway inflammation in a murine model of experimental asthma.
Kerzel S, Päth G, Nockher WA, Quarcoo D, Raap U, Groneberg DA, Dinh QT, Fischer A, Braun A, Renz H. Kerzel S, et al. Am J Respir Cell Mol Biol. 2003 Feb;28(2):170-8. doi: 10.1165/rcmb.4811. Am J Respir Cell Mol Biol. 2003. PMID: 12540484 - A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma.
Caceres AI, Brackmann M, Elia MD, Bessac BF, del Camino D, D'Amours M, Witek JS, Fanger CM, Chong JA, Hayward NJ, Homer RJ, Cohn L, Huang X, Moran MM, Jordt SE. Caceres AI, et al. Proc Natl Acad Sci U S A. 2009 Jun 2;106(22):9099-104. doi: 10.1073/pnas.0900591106. Epub 2009 May 19. Proc Natl Acad Sci U S A. 2009. PMID: 19458046 Free PMC article. - Acid-Sensing Ion Channel 1a Contributes to Airway Hyperreactivity in Mice.
Reznikov LR, Meyerholz DK, Adam RJ, Abou Alaiwa M, Jaffer O, Michalski AS, Powers LS, Price MP, Stoltz DA, Welsh MJ. Reznikov LR, et al. PLoS One. 2016 Nov 7;11(11):e0166089. doi: 10.1371/journal.pone.0166089. eCollection 2016. PLoS One. 2016. PMID: 27820848 Free PMC article. - [The relation between morphologic and functional airway changes in bronchial asthma].
Kips JC. Kips JC. Verh K Acad Geneeskd Belg. 2003;65(4):247-65; discussion 265-9. Verh K Acad Geneeskd Belg. 2003. PMID: 14534940 Review. Dutch.
Cited by
- Transient Receptor Potential Ankyrin 1 (TRPA1) Channel and Neurogenic Inflammation in Pathogenesis of Asthma.
Yang H, Li S. Yang H, et al. Med Sci Monit. 2016 Aug 19;22:2917-23. doi: 10.12659/msm.896557. Med Sci Monit. 2016. PMID: 27539812 Free PMC article. Review. - Silencing Nociceptor Neurons Reduces Allergic Airway Inflammation.
Talbot S, Abdulnour RE, Burkett PR, Lee S, Cronin SJ, Pascal MA, Laedermann C, Foster SL, Tran JV, Lai N, Chiu IM, Ghasemlou N, DiBiase M, Roberson D, Von Hehn C, Agac B, Haworth O, Seki H, Penninger JM, Kuchroo VK, Bean BP, Levy BD, Woolf CJ. Talbot S, et al. Neuron. 2015 Jul 15;87(2):341-54. doi: 10.1016/j.neuron.2015.06.007. Epub 2015 Jun 25. Neuron. 2015. PMID: 26119026 Free PMC article. - Substance P Release by Sensory Neurons Triggers Dendritic Cell Migration and Initiates the Type-2 Immune Response to Allergens.
Perner C, Flayer CH, Zhu X, Aderhold PA, Dewan ZNA, Voisin T, Camire RB, Chow OA, Chiu IM, Sokol CL. Perner C, et al. Immunity. 2020 Nov 17;53(5):1063-1077.e7. doi: 10.1016/j.immuni.2020.10.001. Epub 2020 Oct 23. Immunity. 2020. PMID: 33098765 Free PMC article. - Neuroimmune Interactions in Peripheral Organs.
Klein Wolterink RGJ, Wu GS, Chiu IM, Veiga-Fernandes H. Klein Wolterink RGJ, et al. Annu Rev Neurosci. 2022 Jul 8;45:339-360. doi: 10.1146/annurev-neuro-111020-105359. Epub 2022 Apr 1. Annu Rev Neurosci. 2022. PMID: 35363534 Free PMC article. Review. - Unique Allergic Asthma Phenotypes in Offspring of House Dust Mite-exposed Mice.
Lebold KM, Drake MG, Pincus AB, Pierce AB, Fryer AD, Jacoby DB. Lebold KM, et al. Am J Respir Cell Mol Biol. 2022 Jul;67(1):89-98. doi: 10.1165/rcmb.2021-0535OC. Am J Respir Cell Mol Biol. 2022. PMID: 35363997 Free PMC article.
References
- Anonymous . 2014. Global Strategy for Asthma Management and Prevention. Global Initiative for Asthma (GINA) 2014. Available at: http://www.ginasthma.org/
- Program NAEaP . Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Institutes of Health; 2007. p. 440.
- McFadden ER, Jr, Warren EL. Observations on asthma mortality. Ann Intern Med. 1997;127(2):142–147. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases