Synthesis of bioactive protein hydrogels by genetically encoded SpyTag-SpyCatcher chemistry - PubMed (original) (raw)
Synthesis of bioactive protein hydrogels by genetically encoded SpyTag-SpyCatcher chemistry
Fei Sun et al. Proc Natl Acad Sci U S A. 2014.
Abstract
Protein-based hydrogels have emerged as promising alternatives to synthetic hydrogels for biomedical applications, owing to the precise control of structure and function enabled by protein engineering. Nevertheless, strategies for assembling 3D molecular networks that carry the biological information encoded in full-length proteins remain underdeveloped. Here we present a robust protein gelation strategy based on a pair of genetically encoded reactive partners, SpyTag and SpyCatcher, that spontaneously form covalent isopeptide linkages under physiological conditions. The resulting "network of Spies" may be designed to include cell-adhesion ligands, matrix metalloproteinase-1 cleavage sites, and full-length globular proteins [mCherry and leukemia inhibitory factor (LIF)]. The LIF network was used to encapsulate mouse embryonic stem cells; the encapsulated cells remained pluripotent in the absence of added LIF. These results illustrate a versatile strategy for the creation of information-rich biomaterials.
Keywords: cell fate control; protein biomaterials; stem cell encapsulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
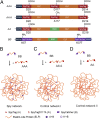
Spy network hydrogels. (A) Genetic constructs for the four protein precursors (AAA, AA′A, AA, and BB). The reactive groups are Asp-20, Asp-117, Asp-214, Lys-57, and Lys-337. AA′A is an Asp-117–to–Ala mutant of AAA, which enables only noncovalent molecular recognition between SpyTag and SpyCatcher at the A′ site. Construct BB contains an internal integrin-binding RGD sequence to facilitate cell adhesion and an MMP-1 cleavage site at the C-terminus to allow matrix remodeling by encapsulated cells. (B_–_D) Schematic illustration of the products formed by mixing protein precursors. AAA+BB leads to the formation of a covalently cross-linked gel, whereas AA′A+BB and AA+BB cannot form covalent molecular networks.
Fig. 2.
Hydrogel formation enabled by SpyTag–SpyCatcher chemistry. (A) Evolution of storage modulus, G′, as a function of time after mixing 10 wt % solutions of AAA+BB, AA′A+BB, and AA+BB at 25 °C. (B) Erosion profiles of the three reaction products (AAA+BB, AA′A+BB, and AA+BB) in water. Aliquots (25 µL) were taken at designated time points. The fraction of protein eroded into water was determined by the Pierce BCA assay. Error bars show SDs from three independent experiments. (C) The Spy network (Top) is formed by mixing 10 wt % aqueous solutions of AAA and BB at a molar ratio of 2:3 (i.e., equimolar A and B). The network swells in water by ∼3,000% after 12 h and remains swollen after 48 h. A mixture of AA′A and BB in aqueous solution (10 wt %; molar ratio, 2:3) yields a physical gel (Middle) that dissolves completely in water after 1 h. Mixing AA and BB (10 wt %; molar ratio, 1:1) yields a product that swells by ∼600% after 12 h and erodes slowly in water (Bottom).
Fig. 3.
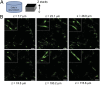
Mouse 3T3 fibroblasts are viable after encapsulation in the Spy network. (A) Schematic of the cell–gel mixture. (B) Confocal fluorescence z-slice micrographs of live (green, calcein AM) and dead (red, ethidium homodimer) cells, showing a highly viable cell population throughout the Spy network (8 wt %). Live cells also exhibit spread morphologies, indicating strong cell adhesion. (Scale bars, 100 µm). Insets are expanded 2.5-fold.
Fig. 4.
Incorporation of mCherry into the Spy network. (A) Genetic construct of the telechelic protein SpyTag-ELP-mCherry-ELP-SpyTag, designated A-mCherry-A. (B) Spy network with embedded mCherry. Protein AAA (10 wt %) was dissolved in an aqueous solution containing 100 µM A-mCherry-A, and mixed with BB (10 wt %; molar ratio of AAA:BB, 2:3). The gel was cured for 6 h at room temperature. Images were taken before and after swelling in water. (C) Encapsulation of 3T3 fibroblasts in the mCherry-Spy network. A representative z-slice is shown. Channel 1 (Ch1) images of the mCherry-Spy network (red); shadows correspond to regions occupied by cells. Channel 2 shows two spreading 3T3/GFP cells (green). Ch1+Ch2 shows an overlay of the Spy network and cells (Scale bars, 10 µm).
Fig. 5.
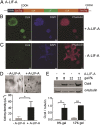
Integration of LIF into the Spy network allows maintenance of mESC pluripotency. (A) Genetic construct of the telechelic protein SpyTag-ELP-MH35-LIF-ELP-SpyTag, designated A-LIF-A. (B and C) Immunocytochemistry and cell image analysis of mESCs encapsulated in Spy networks with (B) and without (C) A-LIF-A show that immobilized LIF promotes Oct4 expression (scale bars, 50 µm). Oct4 staining with anti-Oct4 antibody (green). Nuclear staining with DAPI (blue). F-actin staining with rhodamine phalloidin (red). (D) mESC colony density in 3D cultures formed by 12 wt% Spy networks (− and + A-LIF-A). mESC encapsulation experiments were performed in triplicate. Representative bright-field images are shown (scale bars, 100 µm). Colonies are indicated by arrows. The number of colonies in each gel was determined as described in Experimental Procedures. Data are presented as mean ± SD (n = 3). *Two-tailed P value = 0.0362. (E) Western blot analysis of Oct4 expression (on day 6) in mESCs encapsulated in Spy networks. The relative Oct4 level was determined by dividing the density of each Oct4 band by that of the corresponding α-tubulin (loading-control) band. Oct4 expression in mESCs cultured in 12 wt% Spy network (+ A-LIF-A) is set as 1. Data in the bar graph are presented as mean ± SD (n = 3). **Two-tailed P value = 0.0051. ***Two-tailed P value < 0.0001. Western blots are representative of three independent experiments.
Similar articles
- Engineering Protein Hydrogels Using SpyCatcher-SpyTag Chemistry.
Gao X, Fang J, Xue B, Fu L, Li H. Gao X, et al. Biomacromolecules. 2016 Sep 12;17(9):2812-9. doi: 10.1021/acs.biomac.6b00566. Epub 2016 Aug 17. Biomacromolecules. 2016. PMID: 27477779 - B12-dependent photoresponsive protein hydrogels for controlled stem cell/protein release.
Wang R, Yang Z, Luo J, Hsing IM, Sun F. Wang R, et al. Proc Natl Acad Sci U S A. 2017 Jun 6;114(23):5912-5917. doi: 10.1073/pnas.1621350114. Epub 2017 May 22. Proc Natl Acad Sci U S A. 2017. PMID: 28533376 Free PMC article. - Functional immobilization of signaling proteins enables control of stem cell fate.
Alberti K, Davey RE, Onishi K, George S, Salchert K, Seib FP, Bornhäuser M, Pompe T, Nagy A, Werner C, Zandstra PW. Alberti K, et al. Nat Methods. 2008 Jul;5(7):645-50. doi: 10.1038/nmeth.1222. Epub 2008 Jun 15. Nat Methods. 2008. PMID: 18552855 - Regulation of embryonic stem cell self-renewal and pluripotency by leukaemia inhibitory factor.
Hirai H, Karian P, Kikyo N. Hirai H, et al. Biochem J. 2011 Aug 15;438(1):11-23. doi: 10.1042/BJ20102152. Biochem J. 2011. PMID: 21793804 Free PMC article. Review. - Secrets of a covalent interaction for biomaterials and biotechnology: SpyTag and SpyCatcher.
Reddington SC, Howarth M. Reddington SC, et al. Curr Opin Chem Biol. 2015 Dec;29:94-9. doi: 10.1016/j.cbpa.2015.10.002. Epub 2015 Oct 30. Curr Opin Chem Biol. 2015. PMID: 26517567 Review.
Cited by
- Application of "Click" Chemistry in Biomedical Hydrogels.
Li X, Xiong Y. Li X, et al. ACS Omega. 2022 Oct 12;7(42):36918-36928. doi: 10.1021/acsomega.2c03931. eCollection 2022 Oct 25. ACS Omega. 2022. PMID: 36312409 Free PMC article. Review. - Modular assembly of proteins on nanoparticles.
Ma W, Saccardo A, Roccatano D, Aboagye-Mensah D, Alkaseem M, Jewkes M, Di Nezza F, Baron M, Soloviev M, Ferrari E. Ma W, et al. Nat Commun. 2018 Apr 16;9(1):1489. doi: 10.1038/s41467-018-03931-4. Nat Commun. 2018. PMID: 29662234 Free PMC article. - Advancing Synthetic Hydrogels through Nature-Inspired Materials Chemistry.
Soliman BG, Nguyen AK, Gooding JJ, Kilian KA. Soliman BG, et al. Adv Mater. 2024 Oct;36(42):e2404235. doi: 10.1002/adma.202404235. Epub 2024 Jul 1. Adv Mater. 2024. PMID: 38896849 Review. - Applications of synthetic biology in medical and pharmaceutical fields.
Yan X, Liu X, Zhao C, Chen GQ. Yan X, et al. Signal Transduct Target Ther. 2023 May 11;8(1):199. doi: 10.1038/s41392-023-01440-5. Signal Transduct Target Ther. 2023. PMID: 37169742 Free PMC article. Review. - Modulating the Viscoelastic Properties of Covalently Crosslinked Protein Hydrogels.
Boni R, Regan L. Boni R, et al. Gels. 2023 Jun 12;9(6):481. doi: 10.3390/gels9060481. Gels. 2023. PMID: 37367151 Free PMC article.
References
- Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101(7):1869–1879. - PubMed
- Burdick JA, Murphy WL. Moving from static to dynamic complexity in hydrogel design. Nat Commun. 2012;3:1269. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources