Runx2-Smad signaling impacts the progression of tumor-induced bone disease - PubMed (original) (raw)
. 2015 Mar 15;136(6):1321-32.
doi: 10.1002/ijc.29094. Epub 2014 Aug 5.
Affiliations
- PMID: 25053011
- PMCID: PMC4289427
- DOI: 10.1002/ijc.29094
Runx2-Smad signaling impacts the progression of tumor-induced bone disease
Xuhui Zhang et al. Int J Cancer. 2015.
Abstract
Runx2, a master regulator of osteogenesis, is abnormally expressed in advanced prostate cancer. Here, we addressed Runx2 contribution to formation of prostate cancer-related osteolytic and osteoblastic bone lesions by mediating TGFβ/BMP signaling through direct interaction with Smads. Further, we examined involvement of the Runx2-Smad complex in mediating tumor growth and distal metastasis. To identify Runx2-Smad-specific mechanisms of prostate tumor activity in bone, we generated PC3 prostate cancer cell lines expressing Runx2-WT or one of two mutant proteins (Runx2-HTY and Runx2-ΔC) that each disrupt the Runx2-Smad interaction, either directly through a point mutation or by deletion of the functional C-terminus, respectively. Intratibial tumors generated from these cells revealed that Runx2-WT-expressing cells resulted in predominantly osteolytic disease, whereas cells expressing mutant proteins exhibited tumors with mixed osteolytic/osteoblastic lesions. Extent of bone loss and woven bone formation was assessed by radiography and micro-computed tomography. Bioluminescent imaging showed the presence of labeled prostate cancer cells in the lung at the latest time point examined, with Runx2-WT group exhibiting increased incidence of tumor cells in lung. Notably, disruption of the Runx2-Smad interaction significantly reduced incidence and size of lung tumors. Altered expression of Runx2 target genes involved in invasion, growth, adhesion and metastasis supported our findings. Thus, our studies demonstrate that Runx2 in prostate cancer cells plays a significant role in intratibial prostate cancer-related tumor growth and bone loss through mechanisms mediated by the Runx2-Smad signaling pathway. This work expands upon the potential importance of Runx2 as a therapeutic target in cancer.
Keywords: Runx2; intratibial tumors; metastatic bone disease; osteoblastic/osteolytic disease; prostate cancer.
© 2014 UICC.
Conflict of interest statement
Conflict of Interest Statement: None declared
Figures
Figure 1
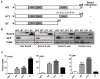
Generation of PC3 cell lines expressing Runx2 and Runx2 mutant proteins. (A) Schematic illustration of the wild-type (WT) and mutant Runx2 (HTY: three essential amino acids mutated to AAA; ΔC: C-terminal deletion mutant) proteins with key regulatory domains (RHD, runt homology domain; NLS, nuclear localization signal; SMID, Smad interaction domain). The ability of the proteins to interact with Smad is indicated to the right of the figure (+, present; −, absent). (B) Western blot showing Runx2 and GFP protein levels in PC3 cells infected with lentiviruses containing GFP only, Runx2 WT or mutant Runx2. Runx2 overexpressing cell lines (non-sorted and 2, 3, 4 weeks post sorting) indicate stable Runx2 WT and mutant Runx2 expression. (C) Gene expression of WT and mutant Runx2 expressing cell lines 5 weeks post sorting. Expression of Runx2, CSF2 and OPN (osteopontin) mRNA was determined by qPCR. Data were normalized to GAPDH and presented relative to the GFP control. Values are mean ± SEM of n=3 experiments analyzed in duplicate. Statistical analysis (Student’s _t_-test) was done by comparing the values of the GFP group with each of the other three groups. *p<0.05, **p<0.01, ***p<0.001.
Figure 2
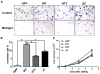
Runx2 proteins modify phenotypic characteristics of PC3 cells. PC3-L cells expressing GFP, Runx2 WT or Runx2 mutant proteins were subjected to invasion and proliferation assays as described in Materials and Methods. (A) Representative images of invasion assays of GFP, Runx2 WT and Runx2 mutant expressing cell lines (HTY and ΔC) are shown. At least three fields of view from each well were photographed (120x). (B) Quantitation of invasion assays: percent invasion rates of the GFP, Runx2 WT and Runx2 mutant expressing cell lines are plotted relative to the GFP control. Rates were calculated based on the number of cells that migrated through matrigel and membrane vs. those which migrated through membrane alone (control) (n=3 per group). Student’s _t-_test was used to calculate significance by comparing the values of the WT group with the other three groups. *p<0.001. (C) Growth curves of the indicated cell lines. Values are mean ± SEM of n=3 experiments analyzed in duplicate. Statistical analysis (Student’s _t_-test) was done by comparing values of the GFP group with the other three groups. *p<0.05.
Figure 3
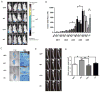
Intratibial tumor size and associated bone erosion vary according to expression of Runx2 WT or mutants in a xenograft model. (A) In vivo imaging of primary tumors. Ventral images of four representative mice (4 of n=6 total) from each group at five weeks post injection are shown. Bioluminescence from the tumors was captured using an IVIS Imaging System. (B) Quantification of primary tumor luminescence intensity (corresponding to panel A). Images were taken at five time points post-intratibial injection as shown. Values are mean ± S.E.M. for n=6 mice. Statistical analysis (Student’s t-test) was done by comparing values of the groups. *p<0.05. (C) Representative images of Toluidine blue staining of the intratibial tumors at sacrifice (5 weeks) to show tumor size and extensive loss of trabecular bone in metaphysis and growth plate in epiphysis in WT and ΔC group. (D) Representative (3 of n=6 total) radiographs of tumor-bearing tibias 2 weeks after injection with GFP, Runx2 or mutant expressing cell lines. Osteolytic lesions are dark areas outlined with white lines representing loss of trabecular bone. (E) Quantification of osteolytic lesions (n=6 mice). Bone erosion areas (as shown in panel D) were quantified by ImageJ. Statistical analysis (Student’s t-test) was done by comparing values of the WT group with the other three groups.*p<0.05, **p<0.01. Arrow, trabecular bone (white areas); T, tumor.
Figure 4
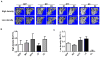
Runx2 promotes lung metastasis. Mice examined in Fig. 3 for bone tumor growth were also examined by Xenogen imaging for presence of tumor cells in lung. (A) Representative images show the extent of lung metastasis in three mice from each group (n=6) 5 weeks after intratibial injection. (B) Quantitation of incidence of lung metastasis in GFP, Runx2 WT and Runx2 mutant groups. (n=6, except for n=5 in ΔC group, as indicated). (C) Quantitative analysis of metastatic cells in lungs by bioluminescence analysis. Statistical analysis (Student’s _t_-test) was done by comparing the values of WT group with the other three groups. *p<0.01.
Figure 5
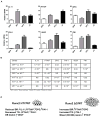
Overexpression of Runx2 and Runx2 mutant proteins modify bone mass in tumor bearing tibias. (A) Two representative micro-CT images of high bone density in tumor-bearing tibias are shown for each group (GFP, WT, HTY and ΔC) at five weeks post-injection by micro-CT. Osteolytic areas are indicated by empty regions in solid bone; mostly mature woven bone is visualized as fine bony projections from the cortical bone. M=mouse. (B), (C) Quantitative micro-CT analysis of high density bone volume fraction (B) and low density bone volume fraction (C) of tumor-bearing tibias. Total volume (TV) is the same in each group. *ANOVA analysis was performed by comparing the values of all the groups. p=0.05. *The contralateral limb CCL is shown for reference to normal bone
Figure 6
Disruption of Runx2-Smad interaction (HTY) and deletion of the Runx2 C-terminus (ΔC) result in altered expression of metastasis-related genes. Adenovirus infected cancer cells expressing Runx2 protein showing changes in molecular markers of osteolytic genes was validated in earlier in vitro studies. (A) The relative expression of Runx2 target genes (IL-11, PTHrP, OPG (osteoprotegrin), PAI-1, VEGF, and FN1) in response to Runx2 WT and mutant protein overexpression is shown. All data were normalized to GAPDH. Values are mean ± SEM of n=3 experiments analyzed in duplicate. ANOVA analysis was done by comparing the values of all four groups. Statistical results are shown in Table S2. (B) The relationship between changes in gene expression and tumor properties in the Runx2 mutant groups compared to Runx2-WT. Arrows indicate up- and down-regulation of the six most highly expressed genes. BR=bone resorption; TG=tumor growth.
Similar articles
- Expression of the IL-11 Gene in Metastatic Cells Is Supported by Runx2-Smad and Runx2-cJun Complexes Induced by TGFβ1.
Zhang X, Wu H, Dobson JR, Browne G, Hong D, Akech J, Languino LR, Stein GS, Lian JB. Zhang X, et al. J Cell Biochem. 2015 Sep;116(9):2098-108. doi: 10.1002/jcb.25167. J Cell Biochem. 2015. PMID: 25808168 Free PMC article. - Runx2 association with progression of prostate cancer in patients: mechanisms mediating bone osteolysis and osteoblastic metastatic lesions.
Akech J, Wixted JJ, Bedard K, van der Deen M, Hussain S, Guise TA, van Wijnen AJ, Stein JL, Languino LR, Altieri DC, Pratap J, Keller E, Stein GS, Lian JB. Akech J, et al. Oncogene. 2010 Feb 11;29(6):811-21. doi: 10.1038/onc.2009.389. Epub 2009 Nov 16. Oncogene. 2010. PMID: 19915614 Free PMC article. - Smad-Runx interactions during chondrocyte maturation.
Leboy P, Grasso-Knight G, D'Angelo M, Volk SW, Lian JV, Drissi H, Stein GS, Adams SL. Leboy P, et al. J Bone Joint Surg Am. 2001;83-A Suppl 1(Pt 1):S15-22. J Bone Joint Surg Am. 2001. PMID: 11263661 Review. - Regulatory roles of Runx2 in metastatic tumor and cancer cell interactions with bone.
Pratap J, Lian JB, Javed A, Barnes GL, van Wijnen AJ, Stein JL, Stein GS. Pratap J, et al. Cancer Metastasis Rev. 2006 Dec;25(4):589-600. doi: 10.1007/s10555-006-9032-0. Cancer Metastasis Rev. 2006. PMID: 17165130 Review.
Cited by
- RUNX2 and the PI3K/AKT axis reciprocal activation as a driving force for tumor progression.
Cohen-Solal KA, Boregowda RK, Lasfar A. Cohen-Solal KA, et al. Mol Cancer. 2015 Jul 25;14:137. doi: 10.1186/s12943-015-0404-3. Mol Cancer. 2015. PMID: 26204939 Free PMC article. Review. - miR-200c and phospho-AKT as prognostic factors and mediators of osteosarcoma progression and lung metastasis.
Berlanga P, Muñoz L, Piqueras M, Sirerol JA, Sánchez-Izquierdo MD, Hervás D, Hernández M, Llavador M, Machado I, Llombart-Bosch A, Cañete A, Castel V, Font de Mora J. Berlanga P, et al. Mol Oncol. 2016 Aug;10(7):1043-53. doi: 10.1016/j.molonc.2016.04.004. Epub 2016 Apr 23. Mol Oncol. 2016. PMID: 27155790 Free PMC article. - The surgical management and treatment of metastatic lesions in the proximal femur: A mini review.
Feng H, Wang J, Xu J, Chen W, Zhang Y. Feng H, et al. Medicine (Baltimore). 2016 Jul;95(28):e3892. doi: 10.1097/MD.0000000000003892. Medicine (Baltimore). 2016. PMID: 27428183 Free PMC article. Review. - A Potential Role of RUNX2- RUNT Domain in Modulating the Expression of Genes Involved in Bone Metastases: An In Vitro Study with Melanoma Cells.
Deiana M, Dalle Carbonare L, Serena M, Cheri S, Mutascio S, Gandini A, Innamorati G, Lorenzi P, Cumerlato M, Bertacco J, Antoniazzi F, Romanelli MG, Mottes M, Zipeto D, Valenti MT. Deiana M, et al. Cells. 2020 Mar 19;9(3):751. doi: 10.3390/cells9030751. Cells. 2020. PMID: 32204402 Free PMC article. - Expression of the IL-11 Gene in Metastatic Cells Is Supported by Runx2-Smad and Runx2-cJun Complexes Induced by TGFβ1.
Zhang X, Wu H, Dobson JR, Browne G, Hong D, Akech J, Languino LR, Stein GS, Lian JB. Zhang X, et al. J Cell Biochem. 2015 Sep;116(9):2098-108. doi: 10.1002/jcb.25167. J Cell Biochem. 2015. PMID: 25808168 Free PMC article.
References
- American Cancer Society. Cancer Facts & Figures 2012. Atlanta: American Cancer Society; 2012. p. 68p.
- Akech J, Wixted JJ, Bedard K, van der Deen M, Hussain S, Guise TA, van Wijnen AJ, Stein JL, Languino LR, Altieri DC, Pratap J, Keller E, et al. Runx2 association with progression of prostate cancer in patients: mechanisms mediating bone osteolysis and osteoblastic metastatic lesions. Oncogene. 2010;29:811–821. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 AR048818/AR/NIAMS NIH HHS/United States
- P01 CA140043/CA/NCI NIH HHS/United States
- P30 CA010815/CA/NCI NIH HHS/United States
- R37 DE012528/DE/NIDCR NIH HHS/United States
- P01 CA082834/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical