Lasting treatment effects in a postmarketing surveillance study of prolonged-release melatonin - PubMed (original) (raw)
Lasting treatment effects in a postmarketing surveillance study of prolonged-release melatonin
Göran Hajak et al. Int Clin Psychopharmacol. 2015 Jan.
Free PMC article
Abstract
Surveillance studies are useful to evaluate how a new medicinal product performs in everyday treatment and how the patient who takes it feels and functions, thereby determining the benefit/risk ratio of the drug under real-life conditions. Prolonged-release melatonin (PRM; Circadin) was approved in Europe for the management of primary insomnia patients age 55 years or older suffering from poor quality of sleep. With traditional hypnotics (e.g. benzodiazepine-receptor agonists), there are concerns about rebound insomnia and/or withdrawal symptoms. We report data from a postmarketing surveillance study in Germany on the effects of 3 weeks of treatment with PRM on sleep in patients with insomnia during treatment and at early (1-2 days) and late (around 2 weeks) withdrawal. In total, 653 patients (597 evaluable) were recruited at 204 sites (mean age 62.7 years, 68% previously treated with hypnotics, 65% women). With PRM treatment, the mean sleep quality (on a scale of 1-5 on which 1 is very good and 5 is very bad) improved from 4.2 to 2.6 and morning alertness improved from 4.0 to 2.5. The improvements persisted over the post-treatment observation period. Rebound insomnia, defined as a one-point deterioration in sleep quality below baseline values, was found in 3.2% (early withdrawal) and 2.0% (late withdrawal). Most of the patients (77%) who used traditional hypnotics before PRM treatment had stopped using them and only 5.6% of naive patients started such drugs after PRM discontinuation. PRM was well tolerated during treatment and the most frequently reported adverse events were nausea (10 patients, 1.5%), dizziness, restlessness and headache (five patients each, <1%). There were no serious adverse events and no adverse events were reported after discontinuation. The persisting treatment effect and very low rebound rate suggest a beneficial role of sleep-wake cycle stabilization with PRM in the treatment of insomnia.
Figures
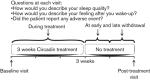
Fig. 1
Study design.
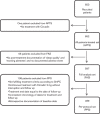
Fig. 2
Overall study patients’ disposition. APTS, all-patients-treated set; FAS, full-analysis set; PPS, per-protocol set; SmPC, summary of product characteristics.
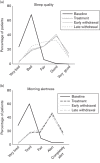
Fig. 3
Sleep quality and morning alertness: (a) percentage of patients with very good, good, fair, bad, and very bad sleep quality at baseline, during treatment, at immediate, and at late withdrawal. (b) Percentage of patients who felt completely alert, alert, fair, tired, and very tired at baseline, during treatment, at immediate, and at late withdrawal.
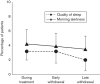
Fig. 4
Rebound insomnia: percentage of patients with a deterioration by at least one point from baseline at immediate and late withdrawal in sleep quality or morning alertness. As a comparison, the percentage of patients deteriorating during ongoing treatment is also shown.
Fig. 5
Use of benzodiazepine and benzodiazepine-like hypnotics: number of patients who used traditional hypnotics before, during the course of, and after prolonged-release melatonin treatment. Percentage of patients using traditional hypnotics (out of 597 in the study) is also shown.
Similar articles
- [Melatonin: Physiological and pharmacological aspects related to sleep: The interest of a prolonged-release formulation (Circadin®) in insomnia].
Quera-Salva MA, Claustrat B. Quera-Salva MA, et al. Encephale. 2018 Dec;44(6):548-557. doi: 10.1016/j.encep.2018.06.005. Epub 2018 Aug 11. Encephale. 2018. PMID: 30107892 Review. French. - Prolonged release melatonin in the treatment of primary insomnia: evaluation of the age cut-off for short- and long-term response.
Wade AG, Crawford G, Ford I, McConnachie A, Nir T, Laudon M, Zisapel N. Wade AG, et al. Curr Med Res Opin. 2011 Jan;27(1):87-98. doi: 10.1185/03007995.2010.537317. Epub 2010 Nov 24. Curr Med Res Opin. 2011. PMID: 21091391 Clinical Trial. - Nightly treatment of primary insomnia with prolonged release melatonin for 6 months: a randomized placebo controlled trial on age and endogenous melatonin as predictors of efficacy and safety.
Wade AG, Ford I, Crawford G, McConnachie A, Nir T, Laudon M, Zisapel N. Wade AG, et al. BMC Med. 2010 Aug 16;8:51. doi: 10.1186/1741-7015-8-51. BMC Med. 2010. PMID: 20712869 Free PMC article. Clinical Trial. - Prolonged-release melatonin improves sleep quality and morning alertness in insomnia patients aged 55 years and older and has no withdrawal effects.
Lemoine P, Nir T, Laudon M, Zisapel N. Lemoine P, et al. J Sleep Res. 2007 Dec;16(4):372-80. doi: 10.1111/j.1365-2869.2007.00613.x. J Sleep Res. 2007. PMID: 18036082 Clinical Trial.
Cited by
- Melatonin's Benefits and Risks as a Therapy for Sleep Disturbances in the Elderly: Current Insights.
Cardinali DP, Brown GM, Pandi-Perumal SR. Cardinali DP, et al. Nat Sci Sleep. 2022 Oct 14;14:1843-1855. doi: 10.2147/NSS.S380465. eCollection 2022. Nat Sci Sleep. 2022. PMID: 36267165 Free PMC article. Review. - Strategies of Functional Foods Promote Sleep in Human Being.
Zeng Y, Yang J, Du J, Pu X, Yang X, Yang S, Yang T. Zeng Y, et al. Curr Signal Transduct Ther. 2014 Dec;9(3):148-155. doi: 10.2174/1574362410666150205165504. Curr Signal Transduct Ther. 2014. PMID: 26005400 Free PMC article. - Eating during the biological night is associated with nausea.
Zitting KM, Isherwood CM, Yuan RK, Wang W, Vujovic N, Münch M, Cain SW, Williams JS, Buxton OM, Czeisler CA, Duffy JF. Zitting KM, et al. Sleep Health. 2024 Feb;10(1S):S144-S148. doi: 10.1016/j.sleh.2023.08.004. Epub 2023 Sep 18. Sleep Health. 2024. PMID: 37730474 - A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials Evaluating the Evidence Base of Melatonin, Light Exposure, Exercise, and Complementary and Alternative Medicine for Patients with Insomnia Disorder.
Baglioni C, Bostanova Z, Bacaro V, Benz F, Hertenstein E, Spiegelhalder K, Rücker G, Frase L, Riemann D, Feige B. Baglioni C, et al. J Clin Med. 2020 Jun 22;9(6):1949. doi: 10.3390/jcm9061949. J Clin Med. 2020. PMID: 32580450 Free PMC article. Review. - Adverse Events Associated with Melatonin for the Treatment of Primary or Secondary Sleep Disorders: A Systematic Review.
Besag FMC, Vasey MJ, Lao KSJ, Wong ICK. Besag FMC, et al. CNS Drugs. 2019 Dec;33(12):1167-1186. doi: 10.1007/s40263-019-00680-w. CNS Drugs. 2019. PMID: 31722088
References
- Czeisler CA, Dumont M, Duffy JF, Steinberg JD, Richardson GS, Brown EN, et al. (1992). Association of sleep-wake habits in older people with changes in output of circadian pacemaker. Lancet 340:933–936. - PubMed
- Garfinkel D, Laudon M, Nof D, Zisapel N. (1995). Improvement of sleep quality in elderly people by controlled-release melatonin. Lancet 346:541–544. - PubMed
- Grossman E, Laudon M, Yalcin R, Zengil H, Peleg E, Sharabi Y, et al. (2006). Melatonin reduces night blood pressure in patients with nocturnal hypertension. Am J Med 119:898–902. - PubMed
- Hajak G, Bandelow B. (1998). Safety and tolerance of zolpidem in the treatment of disturbed sleep: a post-marketing surveillance of 16 944 cases. Int Clin Psychopharmacol 13:157–167. - PubMed
- Hajak G, Clarenbach P, Fischer W, Haase W, Rüther E. (1994). Zopiclone improves sleep quality and daytime well-being in insomniac patients: comparison with triazolam, flunitrazepam and placebo. Int Clin Psychopharmacol 9:251–261. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials