Cyanobacteriochrome SesA is a diguanylate cyclase that induces cell aggregation in Thermosynechococcus - PubMed (original) (raw)
Cyanobacteriochrome SesA is a diguanylate cyclase that induces cell aggregation in Thermosynechococcus
Gen Enomoto et al. J Biol Chem. 2014.
Abstract
Cyanobacteria have unique photoreceptors, cyanobacteriochromes, that show diverse spectral properties to sense near-UV/visible lights. Certain cyanobacteriochromes have been shown to regulate cellular phototaxis or chromatic acclimation of photosynthetic pigments. Some cyanobacteriochromes have output domains involved in bacterial signaling using a second messenger cyclic dimeric GMP (c-di-GMP), but its role in cyanobacteria remains elusive. Here, we characterize the recombinant Tlr0924 from a thermophilic cyanobacterium Thermosynechococcus elongatus, which was expressed in a cyanobacterial system. The protein reversibly photoconverts between blue- and green-absorbing forms, which is consistent with the protein prepared from Escherichia coli, and has diguanylate cyclase activity, which is enhanced 38-fold by blue light compared with green light. Therefore, Tlr0924 is a blue light-activated diguanylate cyclase. The protein's relatively low affinity (10.5 mM) for Mg(2+), which is essential for diguanylate cyclase activity, suggests that Mg(2+) might also regulate c-di-GMP signaling. Finally, we show that blue light irradiation under low temperature is responsible for Thermosynechococcus vulcanus cell aggregation, which is abolished when tlr0924 is disrupted, suggesting that Tlr0924 mediates blue light-induced cell aggregation by producing c-di-GMP. Given our results, we propose the name "sesA (sessility-A)" for tlr0924. This is the first report for cyanobacteriochrome-dependent regulation of a sessile/planktonic lifestyle in cyanobacteria via c-di-GMP.
Keywords: Bacterial Signal Transduction; Cell Aggregation; Cyanobacteria; Cyanobacteriochrome; Cyclic di-GMP (c-di-GMP); Microbiology; Photoreceptor; Sessility; Thermosynechococcus.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
FIGURE 1.
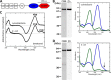
Properties of SesA prepared from the cyanobacterial expression system and E. coli expression system. A, domain composition of SesA deduced by SMART (58) and InterProScan (59). CBS, cystathionine-β-synthase; PAS, Per/ARNT/Sim; GGDEF, diguanylate cyclase domain. B, left, SDS-polyacrylamide gels of Coomassie Brilliant Blue (CBB) (CBB)-stained and Zn2+-enhanced fluorescence (Zn) of SesA expressed in the cyanobacterial system. Right, absorption spectra of native SesA expressed in the cyanobacterial system. When irradiated with blue light, SesA photoconverts to the Pg form (blue line). When irradiated with green light, SesA photoconverts to the Pb form (green line). C, chromophore composition. Light-induced difference spectra of the chromophore (C15-Z minus C15-E) for the acid/urea-denatured Pg form of SesA. Top spectrum, that of SesA expressed in the cyanobacterial system; bottom spectrum, that of SesA expressed in the E. coli system. D, left, SDS-polyacrylamide gels of Coomassie Brilliant Blue (CBB)-stained and Zn2+-enhanced fluorescence (Zn) of SesA expressed in the E. coli system. Right, absorption spectra of native SesA expressed in the E. coli system. When irradiated with blue light, SesA photoconverts to the Pg form (blue line). When irradiated with green light, SesA photoconverts to the Pb form (green line).
FIGURE 2.
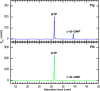
Characterization of SesA as a diguanylate cyclase. SesA was prepared from the cyanobacterial expression system. The reactions included the Pb (bottom panel) or Pg (top panel) forms of SesA, which were incubated for 10 min with GTP. GTP and the product c-di-GMP were separated by HPLC. The mass spectra that proved the second peak to be c-di-GTP are shown in
supplemental Fig. S1
.
FIGURE 3.
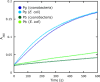
SesA diguanylate cyclase activity. Activity was measured as pyrophosphate production monitored by the change in _A_360 nm. Each reaction was irradiated with blue light (blue) or green light (green) prior to the assay. SesA was prepared from the cyanobacterial (dark blue and green lines) or the E. coli (light blue and green lines) expression system.
FIGURE 4.
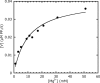
Mg2+ dependence of the SesA diguanylate cyclase activity. The data are fitted to Michaelis-Menten kinetics using the KaleidaGraph fitting program (Synergy Software). The activity (V) is measured as the rate of pyrophosphate production.
FIGURE 5.
Amino acid sequence alignment for T. elongatus and T. vulcanus SesA. The residues that differ in the sequences of the two species are shaded in black. The functionally crucial residues (Cys-499 and Cys-527 in the GAF chromophore-binding domain, Arg-676 and Asp-679 in the GGDEF primary inhibition site, and Gly-685–Phe-689 in the GGDEF active site) are all conserved and are shaded in red. Domains are identified by the bars above the alignment.
FIGURE 6.
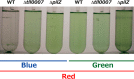
Effects of light color on T. vulcanus cell aggregation at a relatively low temperature of 31 °C. Data are representative of replicated experiments (n = 4). A, photographs of wild-type (WT) T. vulcanus and its Δ_sesA_ mutant (Δ_sesA_) cultured at 31 °C for 24 h and then incubated for 30 min without bubbling in the dark. The cells were cultured with irradiation with blue or green light (5 μmol photons m−2 s−1) in addition to red light (30 μmol photons m−2 s−1), which was needed for photosynthetic growth. Cell aggregation (B) and cell density (C) were reported as the culture OD730 nm as a function of culture time. The definition of the aggregation index refers to “Experimental Procedures.”
FIGURE 7.
Effects of deletion of the PilZ domain of the cellulose synthase Tll0007 on T. vulcanus cell aggregation at a relatively low temperature of 31 °C. Photographs of wild-type (WT) T. vulcanus, its Δ_tll0007_ mutant (Δ_tll0007_), and the mutant whose tll0007 lacks its PilZ domain (Δ_pilZ_) cultured at 31 °C for 3 days and then incubated for 30 min without bubbling in the dark. The cells were cultured with irradiation with blue or green light (5 μmol photons m−2 s−1) in addition to red light (30 μmol photons m−2 s−1), which was needed for photosynthetic growth.
FIGURE 8.
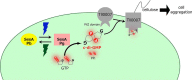
Proposed signaling pathway for SesA. Blue light irradiation photoconverts the Pb form of SesA into the Pg form, which catalyzes the formation of c-di-GMP from two GTP molecules via its diguanylate cyclase activity. When Tll007 binds c-di-GMP at its PilZ domain, it then acts as a cellulose synthase. Extracellular cellulose accumulation results in cell aggregation.
Similar articles
- Three cyanobacteriochromes work together to form a light color-sensitive input system for c-di-GMP signaling of cell aggregation.
Enomoto G, Ni-Ni-Win, Narikawa R, Ikeuchi M. Enomoto G, et al. Proc Natl Acad Sci U S A. 2015 Jun 30;112(26):8082-7. doi: 10.1073/pnas.1504228112. Epub 2015 Jun 15. Proc Natl Acad Sci U S A. 2015. PMID: 26080423 Free PMC article. - Light-Regulated Synthesis of Cyclic-di-GMP by a Bidomain Construct of the Cyanobacteriochrome Tlr0924 (SesA) without Stable Dimerization.
Blain-Hartung M, Rockwell NC, Lagarias JC. Blain-Hartung M, et al. Biochemistry. 2017 Nov 21;56(46):6145-6154. doi: 10.1021/acs.biochem.7b00734. Epub 2017 Nov 8. Biochemistry. 2017. PMID: 29072834 - Thermosynechococcus switches the direction of phototaxis by a c-di-GMP-dependent process with high spatial resolution.
Nakane D, Enomoto G, Bähre H, Hirose Y, Wilde A, Nishizaka T. Nakane D, et al. Elife. 2022 May 10;11:e73405. doi: 10.7554/eLife.73405. Elife. 2022. PMID: 35535498 Free PMC article. - Sensory Perception in Bacterial Cyclic Diguanylate Signal Transduction.
Randall TE, Eckartt K, Kakumanu S, Price-Whelan A, Dietrich LEP, Harrison JJ. Randall TE, et al. J Bacteriol. 2022 Feb 15;204(2):e0043321. doi: 10.1128/JB.00433-21. Epub 2021 Oct 4. J Bacteriol. 2022. PMID: 34606374 Free PMC article. Review. - A Symphony of Cyclases: Specificity in Diguanylate Cyclase Signaling.
Dahlstrom KM, O'Toole GA. Dahlstrom KM, et al. Annu Rev Microbiol. 2017 Sep 8;71:179-195. doi: 10.1146/annurev-micro-090816-093325. Epub 2017 Jun 23. Annu Rev Microbiol. 2017. PMID: 28645224 Free PMC article. Review.
Cited by
- Three cyanobacteriochromes work together to form a light color-sensitive input system for c-di-GMP signaling of cell aggregation.
Enomoto G, Ni-Ni-Win, Narikawa R, Ikeuchi M. Enomoto G, et al. Proc Natl Acad Sci U S A. 2015 Jun 30;112(26):8082-7. doi: 10.1073/pnas.1504228112. Epub 2015 Jun 15. Proc Natl Acad Sci U S A. 2015. PMID: 26080423 Free PMC article. - Protochromic absorption changes in the two-cysteine photocycle of a blue/orange cyanobacteriochrome.
Sato T, Kikukawa T, Miyoshi R, Kajimoto K, Yonekawa C, Fujisawa T, Unno M, Eki T, Hirose Y. Sato T, et al. J Biol Chem. 2019 Dec 6;294(49):18909-18922. doi: 10.1074/jbc.RA119.010384. Epub 2019 Oct 24. J Biol Chem. 2019. PMID: 31649035 Free PMC article. - Hydrophobic Residues near the Bilin Chromophore-Binding Pocket Modulate Spectral Tuning of Insert-Cys Subfamily Cyanobacteriochromes.
Cho SM, Jeoung SC, Song JY, Song JJ, Park YI. Cho SM, et al. Sci Rep. 2017 Jan 17;7:40576. doi: 10.1038/srep40576. Sci Rep. 2017. PMID: 28094296 Free PMC article. - Revealing the origin of multiphasic dynamic behaviors in cyanobacteriochrome.
Wang D, Li X, Zhang S, Wang L, Yang X, Zhong D. Wang D, et al. Proc Natl Acad Sci U S A. 2020 Aug 18;117(33):19731-19736. doi: 10.1073/pnas.2001114117. Epub 2020 Aug 5. Proc Natl Acad Sci U S A. 2020. PMID: 32759207 Free PMC article. - The GGDEF protein Dgc2 suppresses both motility and biofilm formation in the filamentous cyanobacterium Leptolyngbya boryana.
Toida K, Kushida W, Yamamoto H, Yamamoto K, Ishii K, Uesaka K, Kanaly RA, Kutsuna S, Ihara K, Fujita Y, Iwasaki H. Toida K, et al. Microbiol Spectr. 2023 Sep 1;11(5):e0483722. doi: 10.1128/spectrum.04837-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 37655901 Free PMC article.
References
- Möglich A., Yang X., Ayers R. A., Moffat K. (2010) Structure and function of plant photoreceptors. Annu. Rev. Plant Biol. 61, 21–47 - PubMed
- Ikeuchi M., Ishizuka T. (2008) Cyanobacteriochromes: a new superfamily of tetrapyrrole-binding photoreceptors in cyanobacteria. Photochem. Photobiol. Sci. 7, 1159–1167 - PubMed
- Auldridge M. E., Forest K. T. (2011) Bacterial phytochromes: More than meets the light. Crit. Rev. Biochem. Mol. Biol. 46, 67–88 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources