Sialyllactose ameliorates myopathic phenotypes in symptomatic GNE myopathy model mice - PubMed (original) (raw)
Sialyllactose ameliorates myopathic phenotypes in symptomatic GNE myopathy model mice
Takahiro Yonekawa et al. Brain. 2014 Oct.
Abstract
Patients with GNE myopathy, a progressive and debilitating disease caused by a genetic defect in sialic acid biosynthesis, rely on supportive care and eventually become wheelchair-bound. To elucidate whether GNE myopathy is treatable at a progressive stage of the disease, we examined the efficacy of sialic acid supplementation on symptomatic old GNE myopathy mice that have ongoing, active muscle degeneration. We examined the therapeutic effect of a less metabolized sialic acid compound (6'-sialyllactose) or free sialic acid (N-acetylneuraminic acid) by oral, continuous administration to 50-week-old GNE myopathy mice for 30 weeks. To evaluate effects on their motor performance in living mice, spontaneous locomotion activity on a running wheel was measured chronologically at 50, 65, 72 and 80 weeks of age. The size, force production, and pathology of isolated gastrocnemius muscle were analysed at the end point. Sialic acid level in skeletal muscle was also measured. Spontaneous locomotion activity was recovered in 6'-sialyllactose-treated mice, while NeuAc-treated mice slowed the disease progression. Treatment with 6'-sialyllactose led to marked restoration of hyposialylation in muscle and consequently to robust improvement in the muscle size, contractile parameters, and pathology as compared to NeuAc. This is due to the fact that 6'-sialyllactose is longer working as it is further metabolized to free sialic acid after initial absorption. 6'-sialyllactose ameliorated muscle atrophy and degeneration in symptomatic GNE myopathy mice. Our results provide evidence that GNE myopathy can be treated even at a progressive stage and 6'-sialyllactose has more remarkable advantage than free sialic acid, providing a conceptual proof for clinical use in patients.
Keywords: GNE myopathy; amyloid inclusion; distal myopathy with rimmed vacuoles (DMRV)/hereditary inclusion body myopathy (hIBM); hyposialylation; sialyllactose.
© The Author (2014). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures
Figure 1
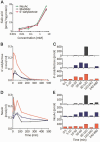
Comparison among compounds to increase cell sialylation and pharmacokinetics of 6’-sialyllactose. (A) Total sialic acid levels in myotubes from a patient with GNE myopathy treated with ManNAc, NeuAc and 6’-sialyllactose. Three compounds were added to condition medium at the concentration shown on the _x_-axis. (B and C) Phamacokinetics of orally administrated 6’-sialyllactose concentration in blood (B) and its excreted amounts in urine (C) before and at 5, 10, 30, 60, 120, 240 and 480 min after its oral administration. (D and E) Profiles of NeuAc concentration in blood (D) and its excreted amounts in urine (E). For the experiment of 6’-sialyllactose pharmacokinetics, three wild-type mice were used. Each colour (grey, blue, and red) denotes one mouse in B–F.
Figure 2
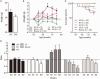
Phenotypes of treated and non-treated GNE myopathy mice. (A) Mean body weights of all GNE myopathy (GM) and littermate (LM) mice at 50 weeks of age. (B) Growth curves. The body weights at 55, 60, 65, 70, and 75 weeks of age are plotted relative to each at 50 weeks of age: GNE myopathy mice with high dose 6’-sialyllactose (HD, red upward closed triangles, n = 8); GNE myopathy mice with low dose 6’-sialyllactose (LD, blue downward closed triangles, n = 10); GNE myopathy mice with NeuAc (green closed diamonds, n = 10); non-treated GNE myopathy mice, non-treated (yellow open circles, n = 18); and control littermates (black closed circles, n = 5). (C) Survival curves: high dose (red), low dose (blue), NeuAc (green), non-treated (yellow) and littermates (black). (D) Spontaneous locomotion activities. The locomotion activities at 65, 72 and 80 weeks of age are plotted relative to those at 50 weeks of age: high dose (dark grey bars, n = 5), low dose (grey bars, n = 7), NeuAc (light grey bars, n = 8), non-treated (white bars, n = 14) and littermates (black bars, n = 14). *P < 0.05, **P < 0.01.
Figure 3
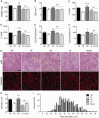
Muscle size and contractile properties of gastrocnemius muscle in GNE myopathy mice after 6’-sialyllactose treatment. (A) Muscle weight. (B) Whole-muscle CSA. (C–F) Contractile properties of the gastrocnemius: (C) isometric force (Pt), (D) tetanic force (Po), (E) specific isometric force (Pt/CSA), and (F) specific tetanic force (Po/CSA). (G) Haematoxylin and eosin staining and immunohistochemistry for laminin-α2 in transverse sections. Arrowheads denote rimmed vacuoles. Scale bar = 50 μm. (H) Mean diameters of myofibres after treatment. Control littermates (LM, black bars, n = 14); non-treated GNE myopathy mice (NT, white bars, n = 15); GNE myopathy mice with high dose 6’-sialyllactose (HD, dark grey bars, n = 5); GNE myopathy mice with low dose 6’-sialyllactose (LD, grey bars, n = 7); GNE myopathy mice with NeuAc (light grey bars, n = 9). (I) Histogram of myofibre diameters. High dose (HD, dark grey bars), low dose (grey bars), NeuAc (light grey bars), non-treated (white bars) and littermates (black bars). The histogram in high dose is shifted to the right, indicating the fibre size is increased after treatment. *P < 0.05, **P < 0.01, ***P < 0.001, ns = not significant.
Figure 4
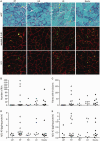
Number of rimmed vacuoles (RVs) and amyloid deposits after 6’-sialyllactose treatment. (A) Modified Gomori trichrome staining of gastrocnemius transverse sections. Arrowheads denote rimmed vacuoles. Immunosignals of amyloid-β1-40 and LC3 are localized within rimmed vacuoles. Scale bar = 50 μm. (B) Number of rimmed vacuoles. (C) Amyloid inclusions. (D and E) Measurement of amyloid-β1-40 (D) and amyloid-β1-42 (E) in muscle by ELISA. Control littermates (LM, closed circles, n = 5); non-treated GNE myopathy mice, (NT, open circles, n = 15); GNE myopathy mice with high dose 6’-sialyllactose (HD, upward closed triangles, n = 5); GNE myopathy mice with low dose 6’-sialyllactose (LD, downward closed triangles, n = 7); GNE myopathy mice with NeuAc (closed diamonds, n = 9).
Figure 5
Total sialic acid levels in blood and tissues. (A) Total sialic acid concentration in blood. (B–D) Sialic acid levels in membranous fraction prepared from skeletal muscle (B), kidney (C), and liver (D). Control littermates (LM, black bars, n = 14); non-treated GNE myopathy mice (NT, white bars, n = 15); GNE myopathy mice with high dose 6’-sialyllactose (HD, dark grey bars, n = 5); GNE myopathy mice with low dose 6’-sialyllactose (LD, grey bars, n = 7); GNE myopathy mice with NeuAc (light grey bars, n = 9). *P < 0.05, **P < 0.01, ns = not significant.
Similar articles
- [Animal model of distal myopathy with rimmed vacuoles/hereditary inclusion body myopathy and preclinical trial with sugar compounds].
Noguchi S, Malicdan MC, Nishino I. Noguchi S, et al. Brain Nerve. 2010 Jun;62(6):601-7. Brain Nerve. 2010. PMID: 20548120 Review. Japanese. - A Gne knockout mouse expressing human GNE D176V mutation develops features similar to distal myopathy with rimmed vacuoles or hereditary inclusion body myopathy.
Malicdan MC, Noguchi S, Nonaka I, Hayashi YK, Nishino I. Malicdan MC, et al. Hum Mol Genet. 2007 Nov 15;16(22):2669-82. doi: 10.1093/hmg/ddm220. Epub 2007 Aug 18. Hum Mol Genet. 2007. PMID: 17704511 - Peracetylated N-acetylmannosamine, a synthetic sugar molecule, efficiently rescues muscle phenotype and biochemical defects in mouse model of sialic acid-deficient myopathy.
Malicdan MC, Noguchi S, Tokutomi T, Goto Y, Nonaka I, Hayashi YK, Nishino I. Malicdan MC, et al. J Biol Chem. 2012 Jan 20;287(4):2689-705. doi: 10.1074/jbc.M111.297051. Epub 2011 Dec 8. J Biol Chem. 2012. PMID: 22157763 Free PMC article. - [Development of therapy for distal myopathy with rimmed vacuoles].
Nishino I, Malicdan MC, Noguchi S. Nishino I, et al. Rinsho Shinkeigaku. 2009 Nov;49(11):852-5. doi: 10.5692/clinicalneurol.49.852. Rinsho Shinkeigaku. 2009. PMID: 20030229 Review. Japanese. - Pharmacokinetics and clinical efficacy of 6'-sialyllactose in patients with GNE myopathy: Randomized pilot trial.
Park YE, Park E, Choi J, Go H, Park DB, Kim MY, Sung NJ, Kim L, Shin JH. Park YE, et al. Biomed Pharmacother. 2023 Dec;168:115689. doi: 10.1016/j.biopha.2023.115689. Epub 2023 Oct 16. Biomed Pharmacother. 2023. PMID: 37852099 Clinical Trial.
Cited by
- 6'-Sialyllactose Enhances Exercise Performance via Increased Muscle Mass and Strength.
Park EJ, Kim LL, Lee JO, Lee HY, Kim YA, Go HR. Park EJ, et al. Nutrients. 2024 Aug 7;16(16):2600. doi: 10.3390/nu16162600. Nutrients. 2024. PMID: 39203737 Free PMC article. - Altered Calcium Permeability of AMPA Receptor Drives NMDA Receptor Inhibition in the Hippocampus of Murine Obesity Models.
Miyagi Y, Fujiwara K, Hikishima K, Utsumi D, Katagiri C, Nishimura M, Takagi H, Ishiuchi S. Miyagi Y, et al. Mol Neurobiol. 2022 Aug;59(8):4902-4925. doi: 10.1007/s12035-022-02834-2. Epub 2022 Jun 3. Mol Neurobiol. 2022. PMID: 35657456 - "Get the Balance Right": Pathological Significance of Autophagy Perturbation in Neuromuscular Disorders.
Castets P, Frank S, Sinnreich M, Rüegg MA. Castets P, et al. J Neuromuscul Dis. 2016 May 27;3(2):127-155. doi: 10.3233/JND-160153. J Neuromuscul Dis. 2016. PMID: 27854220 Free PMC article. Review. - Pharmacological activation of SERCA ameliorates dystrophic phenotypes in dystrophin-deficient mdx mice.
Nogami K, Maruyama Y, Sakai-Takemura F, Motohashi N, Elhussieny A, Imamura M, Miyashita S, Ogawa M, Noguchi S, Tamura Y, Kira JI, Aoki Y, Takeda S, Miyagoe-Suzuki Y. Nogami K, et al. Hum Mol Genet. 2021 May 31;30(11):1006-1019. doi: 10.1093/hmg/ddab100. Hum Mol Genet. 2021. PMID: 33822956 Free PMC article. - Muscle Weakness and Fibrosis Due to Cell Autonomous and Non-cell Autonomous Events in Collagen VI Deficient Congenital Muscular Dystrophy.
Noguchi S, Ogawa M, Malicdan MC, Nonaka I, Nishino I. Noguchi S, et al. EBioMedicine. 2017 Feb;15:193-202. doi: 10.1016/j.ebiom.2016.12.011. Epub 2016 Dec 23. EBioMedicine. 2017. PMID: 28043812 Free PMC article.
References
- ClinicalTrials.gov [Internet] A service of the U.S. National Institute of Health. Available from: http://www.clinicaltrials.gov./ (18 April 2014, date last accessed)
- Drouillard S, Mine T, Kajiwara H, Yamamoto T, Samain E. Efficient synthesis of 6’-sialyllactose, 6,6’-disialyllactose, and 6’-KDO-lactose by metabolically engineered E. coli expressing a multifunctional sialyltransferase from the Photobacterium sp. JT-ISH-224. Carbohydr Res. 2010;345:1394–9. - PubMed
- Eisenberg I, Avidan N, Potikha T, Hochner H, Chen M, Olender T, et al. The UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase gene is mutated in recessive hereditary inclusion body myopathy. Nat Genet. 2001;29:83–7. - PubMed
- Endo S, Morita M, Ueno M, Maeda T, Terabayashi T. Fluorescent labeling of a carboxyl group of sialic acid for MALDI-MS analysis of sialyloligosaccharides and ganglioside. Biochem Biophys Res Commun. 2009;378:890–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases