Principles of targeting endothelial cell metabolism to treat angiogenesis and endothelial cell dysfunction in disease - PubMed (original) (raw)
Review
Principles of targeting endothelial cell metabolism to treat angiogenesis and endothelial cell dysfunction in disease
Jermaine Goveia et al. EMBO Mol Med. 2014 Sep.
Abstract
The endothelium is the orchestral conductor of blood vessel function. Pathological blood vessel formation (a process termed pathological angiogenesis) or the inability of endothelial cells (ECs) to perform their physiological function (a condition known as EC dysfunction) are defining features of various diseases. Therapeutic intervention to inhibit aberrant angiogenesis or ameliorate EC dysfunction could be beneficial in diseases such as cancer and cardiovascular disease, respectively, but current strategies have limited efficacy. Based on recent findings that pathological angiogenesis and EC dysfunction are accompanied by EC-specific metabolic alterations, targeting EC metabolism is emerging as a novel therapeutic strategy. Here, we review recent progress in our understanding of how EC metabolism is altered in disease and discuss potential metabolic targets and strategies to reverse EC dysfunction and inhibit pathological angiogenesis.
Keywords: angiogenesis; endothelial cell dysfunction; metabolism.
© 2014 The Authors. Published under the terms of the CC BY 4.0 license.
Figures
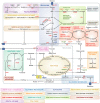
Figure 1. Overview of general EC metabolism
For clarity, not all metabolites and enzymes of the depicted pathways are shown. Abbreviations: 3DG: 3-deoxyglucosone; 3PG: 3-phosphoglycerate; 6PGD: 6-phosphogluconate dehydrogenase; AGE: advanced glycation end-product; AR: aldose reductase; ARG: arginase; ATP: adenosine triphosphate; CPT: carnitine palmitoyltransferase; DHAP: dihydroxyacetone phosphate; eNOS: endothelial nitric oxide synthase; ETC: electron transport chain; F6P: fructose 6-phosphate; F1,6P2: fructose 1,6-bisphosphate; F2,6P2: fructose 2,6 bisphosphate; FA: fatty acid; G6P: glucose 6-phosphate; G6PD: glucose 6-phosphate dehydrogenase; GAPDH: glyceraldehyde 3-phosphate dehydrogenase; GFAT: glutamine-6-phosphate amidotransferase; GlucN6P: glucosamine-6-phosphate; GLS: glutaminase; GLUT: glucose transporter; GS: glutamine synthetase; GSH: glutathione: hCYS: homocysteine; HMG-CoA: hydroxymethylglutaryl coenzyme A; IDH; isocitrate dehydrogenase; LDH: lactate dehydrogenase; MCT: monocarboxylate transporter; ME: malic enzyme; MET: methionine; meTHF: 5.10-methylene-tetrahydrofolate; mTHF: 5-methyltetrahydrofolate; MS: methionine synthetase; NAD: nicotinamide adenine dinucleotide; NADPH: nicotinamide adenine dinucleotide phosphate; NO: nitric oxide; ODC: ornithine decarboxylase; PFK1: phosphofructokinase-1 PFKFB3: 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3; PGK: phosphoglycerate kinase; ROS: reactive oxygen species; RPI: ribose-5-phosphate isomerase; SAH: S-adenosylhomocysteine: SAM: S-adenosylmethionine; TCA cycle: tricarboxylic acid cycle; THF: tetrahydrofolate; TKT: transketolase; UDP-GlcNAc: uridine diphosphate N-acetylglucosamine.
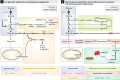
Figure 2. Metabolic pathways implicated in diseases characterized by pathological angiogenesis or hyperproliferative ECs
(A) Angiogenic ECs rely on glycolysis, instead of oxidative metabolism, for ATP production and upregulate PFKFB3 to increase the conversion of glucose into lactate through glycolysis. Lactate is secreted and taken up through lactate transporters. High Lactate influx through MCT1 results in increased intracellular lactate levels that compete with α-ketoglutarate for PHD-2 binding, leading to HIF-1α stabilization and upregulation of pro-angiogenic genes. VEGFR-2 glycosylation is required for galectin-1-induced VEGF-independent signaling. (B) PAH ECs are metabolically characterized by high aerobic glycolysis and low oxidative metabolism. NO production through eNOS is impaired due to upregulation of arginase II and increased oxidative stress due to limited availability of MnSOD. In addition, several enzymes in the pentose phosphate pathway and polyamine biosynthesis pathway are differentially expressed in PAH ECs, but the importance of these findings remains to be determined (B). Green font / bold line: upregulated, red font / broken line: downregulated. For clarity, not all metabolites and enzymes of the depicted pathways are shown. Abbreviations: as in Fig 1. FGF: fibroblast growth factor; HIF: hypoxia-inducible factor; IL: interleukin; PHD: prolyl hydroxylase domain; R5P: ribose-5-phosphate; RPE: ribulose-5-phosphate 3-epimerase; RPIA: ribose-5-phosphate isomerase; Ru5P: ribulose-5-phosphate; SRM: spermidine synthase; VEGFR: vascular endothelial growth factor receptor; Xu5P: xylulose-5-phosphate.
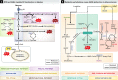
Figure 3. Metabolic pathways implicated in diseases characterized by EC dysfunction
(A) High glucose levels in diabetes pushes glycolytic flux and cause ROS production and AGE formation. (B) Metabolic alterations that cause eNOS dysfunction mediate atherosclerosis pathogenesis. Asymmetric dimethylarginine (ADMA) competes with arginine for binding to eNOS. Arginase expression is increased and eNOS expression is decreased, leading to reduced eNOS activity. 1C metabolism and mevalonate metabolism provide eNOS coupling co-factors and inhibit ROS production. The mevalonate pathway also provides farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP), required for GTPase prenylation. For clarity, not all metabolites and enzymes of the depicted pathways are shown. Green font / bold line: upregulated, red font / broken line: downregulated. Abbreviations: as in Figure 1. BH2: dihydrobiopterin; BH4: tetrahydrobiopterin; ADMA: asymmetric dimethylarginine; CoQ10: coenzyme Q10; DDAH: dimethylarginine dimethylaminohydrolase; DHF: dihydrofolate; DHFR: dihydrofolate reductase; FPP: farnesyl pyrophosphate; GGPP: geranylgeranyl pyrophosphate; GTP: Guanosine triphosphate; HMGCR: hydroxymethylglutaryl coenzyme A reductase; PRMT: protein arginine methyltransferase.
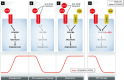
Figure 4. Targeting EC metabolism as an alternative to targeting growth factors in angiogenesis
(A) Vascular endothelial growth factor (VEGF) induces 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3 (PFKFB3) and increases glycolytic flux, required for angiogenesis. (B) Anti-VEGF treatment reduces glycolytic flux and angiogenesis. (C) Increased growth factor signaling through alternative pathways, in this case fibroblast growth factor (FGF), mediates resistance to anti-angiogenic therapy. (D) Pharmacological targeting of PFKFB3 with (3PO) reduces angiogenesis irrespective of growth factor signaling and is therefore possibly less prone to resistance. Abbreviations: as in Figure 1. 3PO: 3-(3-pyridinyl)-1-(4-pyridinyl)-2-propen-1-one; FGF: fibroblast growth factor.
Similar articles
- Endothelial Cell Metabolism in Health and Disease.
Rohlenova K, Veys K, Miranda-Santos I, De Bock K, Carmeliet P. Rohlenova K, et al. Trends Cell Biol. 2018 Mar;28(3):224-236. doi: 10.1016/j.tcb.2017.10.010. Epub 2017 Nov 16. Trends Cell Biol. 2018. PMID: 29153487 Review. - Manipulating Angiogenesis by Targeting Endothelial Metabolism: Hitting the Engine Rather than the Drivers-A New Perspective?
Treps L, Conradi LC, Harjes U, Carmeliet P. Treps L, et al. Pharmacol Rev. 2016 Jul;68(3):872-87. doi: 10.1124/pr.116.012492. Pharmacol Rev. 2016. PMID: 27363442 Review. - The Link Between Angiogenesis and Endothelial Metabolism.
Potente M, Carmeliet P. Potente M, et al. Annu Rev Physiol. 2017 Feb 10;79:43-66. doi: 10.1146/annurev-physiol-021115-105134. Epub 2016 Dec 15. Annu Rev Physiol. 2017. PMID: 27992732 Review. - Endothelial cell plasticity at the single-cell level.
Pasut A, Becker LM, Cuypers A, Carmeliet P. Pasut A, et al. Angiogenesis. 2021 May;24(2):311-326. doi: 10.1007/s10456-021-09797-3. Epub 2021 Jun 1. Angiogenesis. 2021. PMID: 34061284 Free PMC article. - Metabolic Pathways Fueling the Endothelial Cell Drive.
Li X, Kumar A, Carmeliet P. Li X, et al. Annu Rev Physiol. 2019 Feb 10;81:483-503. doi: 10.1146/annurev-physiol-020518-114731. Annu Rev Physiol. 2019. PMID: 30742787 Review.
Cited by
- Oxidized Lipoprotein as a Major Vessel Cell Proliferator in Oxidized Human Serum.
Saito Y, Noguchi N. Saito Y, et al. PLoS One. 2016 Aug 2;11(8):e0160530. doi: 10.1371/journal.pone.0160530. eCollection 2016. PLoS One. 2016. PMID: 27483438 Free PMC article. - Endothelial cell-cardiomyocyte crosstalk in diabetic cardiomyopathy.
Wan A, Rodrigues B. Wan A, et al. Cardiovasc Res. 2016 Aug 1;111(3):172-83. doi: 10.1093/cvr/cvw159. Epub 2016 Jun 10. Cardiovasc Res. 2016. PMID: 27288009 Free PMC article. Review. - Endothelial tip cells in vitro are less glycolytic and have a more flexible response to metabolic stress than non-tip cells.
Yetkin-Arik B, Vogels IMC, Neyazi N, van Duinen V, Houtkooper RH, van Noorden CJF, Klaassen I, Schlingemann RO. Yetkin-Arik B, et al. Sci Rep. 2019 Jul 18;9(1):10414. doi: 10.1038/s41598-019-46503-2. Sci Rep. 2019. PMID: 31320669 Free PMC article. - Anti-Atherosclerotic Effect of Afrocyclamin A against Vascular Smooth Muscle Cells Is Mediated via p38 MAPK Signaling Pathway.
Gu Y, Xiao ZH, Wu J, Guo M, Lv P, Dou N. Gu Y, et al. Cell J. 2021 Jul;23(2):191-198. doi: 10.22074/cellj.2021.7148. Epub 2021 May 26. Cell J. 2021. PMID: 34096220 Free PMC article. - Exosome-mediated metabolic reprogramming: the emerging role in tumor microenvironment remodeling and its influence on cancer progression.
Yang E, Wang X, Gong Z, Yu M, Wu H, Zhang D. Yang E, et al. Signal Transduct Target Ther. 2020 Oct 19;5(1):242. doi: 10.1038/s41392-020-00359-5. Signal Transduct Target Ther. 2020. PMID: 33077737 Free PMC article. Review.
References
- Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008;451:1008–1012. - PubMed
- Atkinson C, Stewart S, Upton PD, Machado R, Thomson JR, Trembath RC, Morrell NW. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation. 2002;105:1672–1678. - PubMed
- van Beijnum JR, Dings RP, van der Linden E, Zwaans BM, Ramaekers FC, Mayo KH, Griffioen AW. Gene expression of tumor angiogenesis dissected: specific targeting of colon cancer angiogenic vasculature. Blood. 2006;108:2339–2348. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources