Homeostatic regulation of adult hippocampal neurogenesis in aging rats: long-term effects of early exercise - PubMed (original) (raw)
Homeostatic regulation of adult hippocampal neurogenesis in aging rats: long-term effects of early exercise
Christina M Merkley et al. Front Neurosci. 2014.
Abstract
Adult neurogenesis is highly responsive to environmental and physiological factors. The majority of studies to date have examined short-term consequences of enhancing or blocking neurogenesis but long-term changes remain less well understood. Current evidence for age-related declines in neurogenesis warrant further investigation into these long-term changes. In this report we address the hypothesis that early life experience, such as a period of voluntary running in juvenile rats, can alter properties of adult neurogenesis for the remainder of the animal's life. The results indicate that the number of proliferating and differentiating neuronal precursors is not altered in runners beyond the initial weeks post-running, suggesting homeostatic regulation of these processes. However, the rate of neuronal maturation and survival during a 4 week period after cell division was enhanced up to 11 months of age (the end of the study period). This study is the first to show that a transient period of physical activity at a young age promotes changes in neurogenesis that persist over the long-term, which is important for our understanding of the modulation of neurogenesis by exercise with age. Functional integration of adult-born neurons within the hippocampus that resist homeostatic regulation with aging, rather than the absolute number of adult-born neurons, may be an essential feature of adult neurogenesis that promotes the maintenance of neural plasticity in old age.
Keywords: adult neurogenesis; aging; dentate gyrus; exercise; hippocampus; homeostasis; plasticity.
Figures
Figure 1
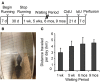
(A) Experimental time line. Male, Long Evans rats were 28 days old at the beginning of the study. Animals were divided into Runners (unlimited wheel access for 30 days) and Controls (non-runners). Four separate batches of Runners and Controls were perfused 1 week, 5 weeks, 6 months, and 9 months following the running period. Animals in the 1 week batch were injected with CldU only, at 7 days prior to perfusion. All remaining batches were injected with CldU at 4 weeks prior to perfusion, and with IdU at 7 days prior to perfusion. (B) Photograph of a 4 week old Male Long Evans rat on the running wheel apparatus. (C) Summary of running behavior (average daily running distance in km; mean ± s.e.m.) for all four batches. ANOVA showed no significant differences in the distance per day between Running cohorts (ANOVA, P = 0.519).
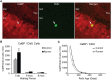
Figure 2
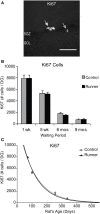
Ki67 cells in the rat DG. (A) Image shows typical Ki67 labeling of proliferating precursors in the subgranular zone (SGZ) bordering between Hilus and granule cell layer (GCL). Full arrowhead indicates one cluster of several nuclei and open arrowhead indicates one single nucleus. Scale bar, 50 μm. (B) Numbers (mean ± s.e.m.) of Ki67 cells per DG. No significant differences were found between Runners and Controls in any cohort (Two-Way ANOVA, P = 0.969). (C) Exponential decay curve fitted to the data for controls (circles) and runners (triangles) show the decay of proliferation with age. In this and subsequent figures the curves were fitted using regression procedure and were significant at P < 0.05. The two parameters were a = 14.376 and b = 0.0090 in Controls and a = 15.418 and b = 0.0098 in Runners. Thus, there were no significant differences between the initial cell numbers (a) or rate constants (b).
Figure 3
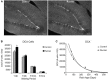
Neuronal differentiation in the rat DG. (A) Fluorescent microscopic images (10×) showing doublecortin (DCX) cells in Runners and Controls at 1 week post-running (10 weeks of age). Scale bar, 100 μm. (B) Number (mean ± s.e.m.) of DCX cells per DG. At 1 week post-running (10 weeks old), Runners had significantly more DCX cells per DG than Controls (Two-Way ANOVA, *P < 0.001). No significant differences were detected between Runners and Controls at any other time point. (C) Exponential decay curve for DCX shows parameters a = 51.650 and b = 0.0077 in Controls and a = 67.680 and b = 0.0089 in Runners. This is consistent with the higher initial cell number at younger ages and slightly faster decay in runners compared with controls.
Figure 4
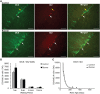
Neuronal differentiation and survival in 1 week old neurons. (A) Confocal microscopic images (40×) showing DCX and CldU labeled cells in the DG of 10 week old Control (top panels) and Runner (bottom panels). White arrows indicate double-labeled cells, and yellow arrow indicates single-labeled CldU cell. Scale bar, 50 um. (B) Numbers (mean ± s.e.m.) of dual-labeled DCX/IdU cells. Runners in the 1 week cohort (10 weeks of age) showed significantly more dual-labeled cells than Controls (Two-Way ANOVA, *P = 0.007). There were no differences between Runners and Controls in any other group. (C) Exponential decay curve for DCX/IdU shows parameters a = 22.120 and b = 0.0206 in Controls and a = 58.070 and b = 0.0301 in Runners. The data from the regression analysis is consistent with higher initial cell numbers in Runners early on, but faster decay with age.
Figure 5
Survival and neuronal maturation. (A) Confocal microscopic images (40×, 1 μm thickness) showing CaBP and CldU-labeled cells in the GCL. White arrow indicates dual-labeled CaBP/CldU cell in the GCL. Scale bar, 10 um. (B) Number (mean ± s.e.m.) of dual-labeled cells per DG. Runners in the 5 week cohort (14 weeks of age) showed significantly more dual-labeled cells than Controls (Two-Way ANOVA, *P < 0.001). (C) Exponential decay curve for CaBP/CldU shows parameters a = 7775 and b = 0.0164 in Controls and a = 12.233 and b = 0.0166 in Runners. Regression analysis suggests substantially higher initial cell number in runners but virtually identical decay rates in runners and controls. Hence, persistent change in cell maturation and survival with aging.
Similar articles
- Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation.
Sahay A, Scobie KN, Hill AS, O'Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Sahay A, et al. Nature. 2011 Apr 28;472(7344):466-70. doi: 10.1038/nature09817. Epub 2011 Apr 3. Nature. 2011. PMID: 21460835 Free PMC article. - Adult-Born Hippocampal Neurons Undergo Extended Development and Are Morphologically Distinct from Neonatally-Born Neurons.
Cole JD, Espinueva DF, Seib DR, Ash AM, Cooke MB, Cahill SP, O'Leary TP, Kwan SS, Snyder JS. Cole JD, et al. J Neurosci. 2020 Jul 22;40(30):5740-5756. doi: 10.1523/JNEUROSCI.1665-19.2020. Epub 2020 Jun 22. J Neurosci. 2020. PMID: 32571837 Free PMC article. - A combination of running and memantine increases neurogenesis and reduces activation of developmentally-born dentate granule neurons in rats.
Cahill SP, Martinovic A, Cole JD, Seib DR, Snyder JS. Cahill SP, et al. Behav Brain Res. 2019 Oct 17;372:112005. doi: 10.1016/j.bbr.2019.112005. Epub 2019 Jun 2. Behav Brain Res. 2019. PMID: 31167109 - Running in laboratory and wild rodents: differences in context sensitivity and plasticity of hippocampal neurogenesis.
Klaus F, Amrein I. Klaus F, et al. Behav Brain Res. 2012 Feb 14;227(2):363-70. doi: 10.1016/j.bbr.2011.04.027. Epub 2011 Apr 27. Behav Brain Res. 2012. PMID: 21549157 Review. - Social Cues, Adult Neurogenesis, and Reproductive Behavior.
Peretto P, Paredes RG. Peretto P, et al. In: Mucignat-Caretta C, editor. Neurobiology of Chemical Communication. Boca Raton (FL): CRC Press/Taylor & Francis; 2014. Chapter 13. In: Mucignat-Caretta C, editor. Neurobiology of Chemical Communication. Boca Raton (FL): CRC Press/Taylor & Francis; 2014. Chapter 13. PMID: 24830028 Free Books & Documents. Review.
Cited by
- Spatial transcriptomic analysis of adult hippocampal neurogenesis in the human brain.
Simard S, Rahimian R, Davoli MA, Théberge S, Matosin N, Turecki G, Nagy C, Mechawar N. Simard S, et al. J Psychiatry Neurosci. 2024 Oct 16;49(5):E319-E333. doi: 10.1503/jpn.240026. Print 2024 Sep-Oct. J Psychiatry Neurosci. 2024. PMID: 39414359 Free PMC article. - Analysis of hippocampal synaptic function in a rodent model of early life stress.
Wilkinson MP, Robinson ESJ, Mellor JR. Wilkinson MP, et al. Wellcome Open Res. 2024 Jun 10;9:300. doi: 10.12688/wellcomeopenres.22276.1. eCollection 2024. Wellcome Open Res. 2024. PMID: 39221440 Free PMC article. - Mind the ramp: Association between early life ramp use and spatial cognition in laying hen pullets.
Johny A, Janczak AM, Nordgreen J, Toscano MJ, Stratmann A. Johny A, et al. PLoS One. 2024 Apr 26;19(4):e0302454. doi: 10.1371/journal.pone.0302454. eCollection 2024. PLoS One. 2024. PMID: 38669289 Free PMC article. - Age-related changes in mice behavior and the contribution of lipocalin-2.
Ferreira AC, Sousa N, Sousa JC, Marques F. Ferreira AC, et al. Front Aging Neurosci. 2023 Apr 24;15:1179302. doi: 10.3389/fnagi.2023.1179302. eCollection 2023. Front Aging Neurosci. 2023. PMID: 37168715 Free PMC article. - Changes of Development from Childhood to Late Adulthood in Rats Tracked by Urinary Proteome.
Pan X, Liu Y, Bao Y, Gao Y. Pan X, et al. Mol Cell Proteomics. 2023 Jun;22(6):100539. doi: 10.1016/j.mcpro.2023.100539. Epub 2023 Mar 31. Mol Cell Proteomics. 2023. PMID: 37004987 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources