Integrated action of pheromone signals in promoting courtship behavior in male mice - PubMed (original) (raw)
Integrated action of pheromone signals in promoting courtship behavior in male mice
Sachiko Haga-Yamanaka et al. Elife. 2014.
Abstract
The mammalian vomeronasal organ encodes pheromone information about gender, reproductive status, genetic background and individual differences. It remains unknown how pheromone information interacts to trigger innate behaviors. In this study, we identify vomeronasal receptors responsible for detecting female pheromones. A sub-group of V1re clade members recognizes gender-identifying cues in female urine. Multiple members of the V1rj clade are cognate receptors for urinary estrus signals, as well as for sulfated estrogen (SE) compounds. In both cases, the same cue activates multiple homologous receptors, suggesting redundancy in encoding female pheromone cues. Neither gender-specific cues nor SEs alone are sufficient to promote courtship behavior in male mice, whereas robust courtship behavior can be induced when the two cues are applied together. Thus, integrated action of different female cues is required in pheromone-triggered mating behavior. These results suggest a gating mechanism in the vomeronasal circuit in promoting specific innate behavior.DOI: http://dx.doi.org/10.7554/eLife.03025.001\.
Keywords: GCaMP; imaging; innate behavior; olfactory; pheromone; vomeronasal.
Copyright © 2014, Haga-Yamanaka et al.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures
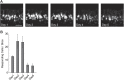
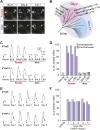
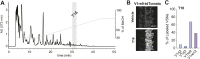
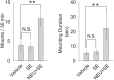
Figure 1.. Sulfated estrogens mimic the activity of estrus signal in urine.
(A) The number (left) and duration (right) of mounting behavior of sexually naïve males toward ovariectomized females painted with vehicle (n = 16), non-estrus urine (NEU; n = 14) or estrus urine (EU; n = 22). EU and NEU were collected 2 and 4 days, respectively, after PMSG injection to induce estrus. Error bars, SEM; *p<0.05 (Mann–Whitney test). (B) Traces showing GCaMP2 responses of a representative cell to urine samples collected from females 1 to 5 days after PMSG injection and SEs. Arrows indicate the onset of stimulus delivery. (C) Representative images of the VNO slice response pattern to E1050 (magenta) and urine (green). Scale bar, 50 μm. (D) Bar graph showing the percentage of SE-activated VSNs (n = 86; 3 slices) that are also activated by female urine samples. (E) Venn diagrams showing the overlap between VSNs responding to SEs and those activated by EU or NEU. DOI:
http://dx.doi.org/10.7554/eLife.03025.003
Figure 1—figure supplement 1.. Activation of the VSNs by female mouse urine.
(A) Representative images of the VNO slice response pattern to urine samples collected from females 1 to 5 days after PMSG injection. Scale bar, 50 μm. (B) Bar graph showing the number of responding cells to urine samples collected from females 1 to 5 days after PMSG injection in each slice (3 slices). Error bars, SEM. DOI:
http://dx.doi.org/10.7554/eLife.03025.004
Figure 1—figure supplement 2.. Activation of the VSNs by sulfated estrogen E1050 and E1103.
(A) Representative images of a GCaMP2 VNO slice responding to vehicle, 100 nM E1050 and E1103. Bright cells are the activated neurons. VSNs responding to E1050 (magenta) or E1103 (green) are color-coded in the right panel to show overlap (white). Scale bar, 50 μm. (B) Venn diagram of the overlap of VSNs responding to 100 nM E1050 and E1103. (C) Representative images of the VNO slice response pattern to E1103 and urine samples collected from females 2 or 4 days after PMSG injection. VSNs responding to E1103 (magenta) or urine samples (green) are color-coded to show overlap (white). Scale bar, 50 μm. DOI:
http://dx.doi.org/10.7554/eLife.03025.005
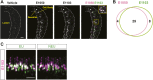
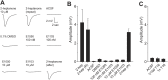
Figure 2.. VSNs responding to estrus signal express V1rj receptors.
(A) Representative images of the single-cell isolation procedure. Arrowheads indicate a responding cell illuminated under fluorescence (left), which is aspirated into a micro-capillary (arrow) under bright field illumination (right). Scale bar, 20 μm. (B) Schematic illustration of single-cell degenerate RT-PCR procedure. (C) Tables showing the receptor genes cloned from individual E1050-responding (left) and E1103-responding VSNs (right). Members of V1rj clade are indicated in red. (D) Enlarged view of phylogenetic tree showing the V1rj clade. Receptors cloned from SE-responding VSNs are indicated in red. Numbers in parentheses indicate the number of the VSNs that expressed the receptor out of the total number of SE-responding VSNs profiled. DOI:
http://dx.doi.org/10.7554/eLife.03025.006
Figure 2—figure supplement 1.. Members of V1rj group receptors are homologous to each other.
(A) Alignment of amino acid sequences of the members of V1Rj group receptors found in sulfated estrogens responding cells. Identical residues are indicated by asterisks. The potential transmembrane (TM) domains are highlighted in gray. (B) Amino acid similarities and identities among the members of V1rj group receptors. DOI:
http://dx.doi.org/10.7554/eLife.03025.007
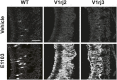
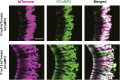
Figure 3.. V1rj receptors selectively respond to sulfated estrogens.
(A) Schematic illustration of transgenic alleles that induce ectopic V1rj2- or V1rj3-expression in the VNO: (i) Gγ8-tTA allele drives tTA expression in immature VSNs; (ii) Knock-in OMP-IRES-tTA (OIVT) allele drives tTA expression in mature VSNs; (iii) tetO-V1rj2/3-tdTomato allele that allows bicistronic expression of V1rj2 or V1rj3 with tdTomato; (iv) tetO-GCaMP2 allele. (B) Representative images of VNO slices from Gγ8-tTA;OIVT;tetO-V1rj2-tdTomato;tetO-GCaMP2 (top) and Gγ8-tTA;OIVT;tetO-V1rj3-tdTomato;tetO-GCaMP2 (bottom) mice. D: dendrite; CB: cell body. Scale bar, 50 μm. (C) Representative images of GCaMP2 VNO slices from the control (left), V1rj2- (middle) and V1rj3-tdTomato-expressing (right) mice responding to vehicle (top) or 100 nM E1050 (bottom). Scale bar, 50 μm. (D and E) Traces showing GCaMP2 responses of two representative V1rj2 (D) or V1rj3 (E). Response of tdTomato-expressing cells to different concentrations of E1050 (top) and E1103 (bottom). Arrows indicate the onset of stimulus delivery. (F and G) Bar graph showing the normalized response amplitude of V1rj2 (F; n = 247) or V1rj3 (G; n = 207) VSNs to a set of sulfated steroid compounds. Error bars, SEM. DOI:
http://dx.doi.org/10.7554/eLife.03025.008
Figure 3—figure supplement 1.. E1103 activates V1rj2/3-expressing VSNs.
Representative images of GCaMP2 VNO slices from the control (left), V1rj2-tdTomato-expressing (middle) and V1rj3-tdTomato-expressing (right) mice responding to vehicle (top) or 100 nM E1103 (bottom). Bright cells are the activated neurons. The control is the same slice as in Figure 3C. Scale bar, 50 μm. DOI:
http://dx.doi.org/10.7554/eLife.03025.009
Figure 4.. V1rj receptors selectively respond to estrus urinary cues.
(A) Traces showing GCaMP2 responses of a representative V1rj2-tdTomato-expressing cell (top) and a V1rj3-tdTomato-expressing cell (bottom) to urine samples collected from females 1 to 5 days after PMSG injection. (B) Bar graph showing normalized response amplitude of V1rj2 (n = 43) or V1rj3 (n = 77) VSNs labeled by both GCaMP2 and tdTomato. Error bars: SEM. DOI:
http://dx.doi.org/10.7554/eLife.03025.010
Figure 4—figure supplement 1.. Urine from estrous females in natural estrus cycle activates V1rj3-expressing VSNs.
Bar graph showing normalized response amplitude of V1j3-expressing VSNs to urine from estrous and diestrous females in the natural estrus cycle. Error bars, SEM. DOI:
http://dx.doi.org/10.7554/eLife.03025.011
Figure 5.. V1re-Chr.7 group receptors recognize female-specific gender signals.
(A) Representative images of VSNs responding to urine samples from either females (top) or males (bottom) of different mouse strains. White arrow-heads indicate a Female Urine Specific Cell (FUSC). Scale bar, 20 μm. (B) Enlarged view of the V1re clade of the phylogenetic tree of the V1r family. Receptors located on Chr.7 and Chr.17 are circled in pink and light blue, respectively. Receptors cloned from FUSCs are indicated in bold font. The numbers in parentheses indicate the number of the VSNs that express the receptor over the total numbers of FUSCs examined. (C) Traces showing the GCaMP2 responses of a representative V1re9- (top) and a V1re12-tdTomato-expressing cell (bottom) to female and male urine samples from multiple mouse strains. (D) Bar graph showing the percentage of V1re9 (n = 195) or V1re12 (n = 201) VSNs responding to urine samples from various strains of males and females. (E) Traces showing the GCaMP2 responses of a representative V1re9- (top) and a V1re12-tdTomato-expressing cell (bottom) to urine samples from females 1 to 5 days after PMSG injection. (F) Bar graph showing the percentage of V1re9 (n = 120) or V1re12 (n = 209) VSNs responding to female urine samples. DOI:
http://dx.doi.org/10.7554/eLife.03025.012
Figure 5—figure supplement 1.. Members of V1re-Chr.7 group receptors are homologous to each other.
(A) Alignment of amino acid sequences of the members of V1re-Chr.7 group receptors found in FUSCs. Identical residues are indicated by asterisks. The potential transmembrane (TM) domains are highlighted in gray. (B) Amino acid similarities and identities of the members of V1re-Chr.7 group receptors. DOI:
http://dx.doi.org/10.7554/eLife.03025.013
Figure 5—figure supplement 2.. V1re9/12-expressing VSNs express GCaMP2.
Representative images of VNO of Gγ8-tTA;OIVT;tetO-V1re9-tdTomato;tetO-GCaMP2 (top) and Gγ8-tTA;OIVT;tetO-V1re12-tdTomato;tetO-GCaMP2 (bottom) mice. Scale bar, 50 μm. DOI:
http://dx.doi.org/10.7554/eLife.03025.014
Figure 5—figure supplement 3.. Urine sample from ovariectomized females activates V1re9/e12-expressing cells.
(A) Bar graph showing normalized response amplitude of V1e9 (n = 133) or V1re12 (n = 137) VSNs labeled by both GCaMP2 and tdTomato to urine from estrus (EU) and ovariectomized (OVX) females. (B) Bar graph showing the percentage of V1re9 (n = 371) or V1re12 (n = 221) VSNs responding to urine samples from estrus and ovariectomized females. Error bars, SEM. DOI:
http://dx.doi.org/10.7554/eLife.03025.015
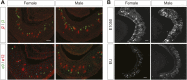
Figure 6.. The female cues are detected in male and female VNOs.
(A) Double in situ hybridization of VNO slices. Top row: confocal images showing cells expressing V1rj2 (red) and V1rj3 (green) in VNO sections obtained from female (left) and male (right) mice. Bottom row: confocal images showing cells labeled by V1re9 (green) and V1re12 (red) probes in VNO sections from female (left) and male (right) mice. (B) Representative images of GCaMP2 VNO slices from female (left) and male (right) mice responding to 100 nM E1050 (top) and estrus urine (bottom). Scale bars, 50 μm. DOI:
http://dx.doi.org/10.7554/eLife.03025.016
Figure 7.. T16 fraction contains the female-specific gender cue.
(A) Chromatogram of HPLC purification using a C18 column. The light gray bar indicates the T16 fraction that activates the V1re9 and V1re12 receptors. (B) A representative image of GCaMP2 VNO slices from a V1re9-tdTomato-expressing mouse responding to the T16 fraction. Scale bar, 50 μm. (C) Bar graph showing the percentage of V1rj2 (n = 172), V1rj3 (n = 243), V1re9 (n = 189) or V1re12 (n = 205) VSNs responding to the T16 fraction. DOI:
http://dx.doi.org/10.7554/eLife.03025.017
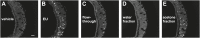
Figure 7—figure supplement 1.. Acetone fraction from XAD4 resins retains VNO-stimulatory activity.
Representative images of a GCaMP2 VNO slice in response to vehicle (A), estrus urine (EU; B), flow-through (C), water (D) and acetone (E) fractions. Scale bar, 50 μm. DOI:
http://dx.doi.org/10.7554/eLife.03025.018
Figure 8.. Sulfated estrogens and T16 fraction do not activate the main olfactory system.
(A) Traces showing EOG responses to 2-heptanone, E1050 and E1103. (B and C) Bar graphs showing the mean amplitude of EOG responses to 2-heptanone, E1050 and E1103 (B) and the T16 fraction (C). Error bars: SEM (n = 3 mice). DOI:
http://dx.doi.org/10.7554/eLife.03025.019
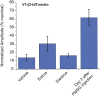
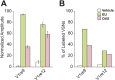
Figure 9.. Combined female and estrus cues are sufficient to promote mounting behavior.
(A) The number (left), duration (middle) and latency (right) of mounting behavior of naïve males toward females painted with vehicle, T16 (n = 13), SE (n = 10), T16+SE (n = 13) or EU samples. The data presented with white bars are the same as in Figure 1. Error bars: SEM; *p<0.05 (Mann–Whitney test). (B and C) Schematic illustrations of two alternative models of neural circuits that integrate the female and estrus signals. DOI:
http://dx.doi.org/10.7554/eLife.03025.020
Figure 9—figure supplement 1.. Sulfated estrogens promote courtship in conjunction with NEU.
The number (left panel), duration (middle panel) and latency (right panel) of mounting behavior of sexually naïve males toward the females painted with either vehicle, SE or non-estrus urine (NEU)+SE. The vehicle control is the same as in Figure 1A. Error bars, SEM; n = 10 for SE, n = 16 for NEU+SE; **p<0.01. DOI:
http://dx.doi.org/10.7554/eLife.03025.021
Similar articles
- Impaired pheromone detection and abnormal sexual behavior in female mice deficient for ancV1R.
Kondo H, Iwata T, Sato K, Koshiishi R, Suzuki H, Murata K, Spehr M, Touhara K, Nikaido M, Hirota J. Kondo H, et al. Curr Biol. 2025 Jan 6;35(1):21-35.e8. doi: 10.1016/j.cub.2024.10.077. Epub 2024 Nov 21. Curr Biol. 2025. PMID: 39577426 - Male pheromone protein components activate female vomeronasal neurons in the salamander Plethodon shermani.
Wirsig-Wiechmann CR, Houck LD, Wood JM, Feldhoff PW, Feldhoff RC. Wirsig-Wiechmann CR, et al. BMC Neurosci. 2006 Mar 22;7:26. doi: 10.1186/1471-2202-7-26. BMC Neurosci. 2006. PMID: 16553953 Free PMC article. - Encoding gender and individual information in the mouse vomeronasal organ.
He J, Ma L, Kim S, Nakai J, Yu CR. He J, et al. Science. 2008 Apr 25;320(5875):535-8. doi: 10.1126/science.1154476. Science. 2008. PMID: 18436787 Free PMC article. - Pheromone reception in mammals.
Bigiani A, Mucignat-Caretta C, Montani G, Tirindelli R. Bigiani A, et al. Rev Physiol Biochem Pharmacol. 2005;154:1-35. doi: 10.1007/s10254-004-0038-0. Rev Physiol Biochem Pharmacol. 2005. PMID: 15800771 Review. - Refining the dual olfactory hypothesis: pheromone reward and odour experience.
Martínez-García F, Martínez-Ricós J, Agustín-Pavón C, Martínez-Hernández J, Novejarque A, Lanuza E. Martínez-García F, et al. Behav Brain Res. 2009 Jun 25;200(2):277-86. doi: 10.1016/j.bbr.2008.10.002. Epub 2008 Oct 11. Behav Brain Res. 2009. PMID: 18977394 Review.
Cited by
- Hormonal Modulation of Pheromone Detection Enhances Male Courtship Success.
Lin HH, Cao DS, Sethi S, Zeng Z, Chin JSR, Chakraborty TS, Shepherd AK, Nguyen CA, Yew JY, Su CY, Wang JW. Lin HH, et al. Neuron. 2016 Jun 15;90(6):1272-1285. doi: 10.1016/j.neuron.2016.05.004. Epub 2016 Jun 2. Neuron. 2016. PMID: 27263969 Free PMC article. - Sex steroid hormone synthesis, metabolism, and the effects on the mammalian olfactory system.
Abaffy T, Lu HY, Matsunami H. Abaffy T, et al. Cell Tissue Res. 2023 Jan;391(1):19-42. doi: 10.1007/s00441-022-03707-9. Epub 2022 Nov 19. Cell Tissue Res. 2023. PMID: 36401093 Free PMC article. Review. - Hypothalamic representation of the imminence of predator threat detected by the vomeronasal organ in mice.
Nguyen QAT, Rocha A, Chhor R, Yamashita Y, Stadler C, Pontrello C, Yang H, Haga-Yamanaka S. Nguyen QAT, et al. Elife. 2024 Oct 16;12:RP92982. doi: 10.7554/eLife.92982. Elife. 2024. PMID: 39412856 Free PMC article. - Male mice adjust courtship behavior in response to female multimodal signals.
Ronald KL, Zhang X, Morrison MV, Miller R, Hurley LM. Ronald KL, et al. PLoS One. 2020 Apr 2;15(4):e0229302. doi: 10.1371/journal.pone.0229302. eCollection 2020. PLoS One. 2020. PMID: 32241020 Free PMC article. - Neural basis for pheromone signal transduction in mice.
Murata K, Itakura T, Touhara K. Murata K, et al. Front Neural Circuits. 2024 Apr 29;18:1409994. doi: 10.3389/fncir.2024.1409994. eCollection 2024. Front Neural Circuits. 2024. PMID: 38742089 Free PMC article. Review.
References
- Brechbuhl J, Moine F, Klaey M, Nenniger-Tosato M, Hurni N, Sporkert F, Giroud C, Broillet MC. 2013. Mouse alarm pheromone shares structural similarity with predator scents. Proceedings of the National Academy of Sciences of the United States of America 110:4762–4767. doi: 10.1073/pnas.1214249110 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases