Long-term outcome of anti-glomerular basement membrane antibody disease treated with immunoadsorption - PubMed (original) (raw)
Long-term outcome of anti-glomerular basement membrane antibody disease treated with immunoadsorption
Peter Biesenbach et al. PLoS One. 2014.
Abstract
Background: Anti-glomerular basement membrane (GBM) antibody disease may lead to acute crescentic glomerulonephritis with poor renal prognosis. Current therapy favours plasma exchange (PE) for removal of pathogenic antibodies. Immunoadsorption (IAS) is superior to PE regarding efficiency of antibody-removal and safety. Apart from anecdotal data, there is no systemic analysis of the long-term effects of IAS on anti-GBM-disease and antibody kinetics.
Objective: To examine the long-term effect of high-frequency IAS combined with standard immunosuppression on patient and renal survival in patients with anti-GBM-disease and to quantify antibody removal and kinetics through IAS.
Design: Retrospective review of patients treated with IAS for anti-GBM-antibody disease confirmed by biopsy and/or anti-GBM-antibodies.
Setting: University Hospital of Vienna, Austria.
Participants: 10 patients with anti-GBM-disease treated with IAS.
Measurements: Patient and renal survival, renal histology, anti-GBM-antibodies.
Results: Anti-GBM-antibodies were reduced by the first 9 IAS treatments (mean number of 23) to negative levels in all patients. Renal survival was 40% at diagnosis, 70% after the end of IAS, 63% after one year and 50% at the end of observation (mean 84 months, range 9 to 186). Dialysis dependency was successfully reversed in three of six patients. Patient survival was 90% at the end of observation.
Conclusion: IAS efficiently eliminates anti-GBM-antibodies suggesting non-inferiority to PE with regard to renal and patient survival. Hence IAS should be considered as a valuable treatment option for anti-GBM-disease, especially in patients presenting with a high percentage of crescents and dialysis dependency due to an unusual high proportion of responders.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
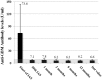
Figure 1. Anti-GBM antibody levels from start of immunoadsorption until end of observation.
Mean values ± standard deviation of all patients measured with ELISA depicted. Grey area denotes negativity of the assay.
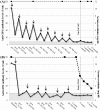
Figure 2. Anti-GBM antibody kinetics and renal function in patient #7 (2a) and patient #10 (2b).
Antibody values at diagnosis, before and after IAS, 12 hours in between sessions and up to 6 weeks; arrows depict daily IAS sessions. Grey area depicts negativity of the assay.
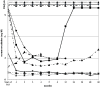
Figure 3. Dynamics of serum-creatinine of individual patients.
Observation period from start of IAS until 5 years. White coloured time points depict positive anti-GBM antibody testing.
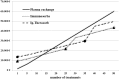
Figure 4. Cumulative costs of immunoadsorption and plasma exchange.
Circles and triangles depict earliest timepoints of adsorber change due to reduced adsorption capacity (every 25 treatments for Immunosorba, every 35 treatments for Globaffin).
Similar articles
- Effectiveness of Plasmapheresis in a Patient with Anti-glomerular Basement Membrane Antibody Glomerulonephritis with Advanced Kidney Dysfunction.
Usui T, Kai H, Noguchi K, Morito N, Usui J, Saito C, Uesugi N, Nagata M, Yamagata K. Usui T, et al. Intern Med. 2017 Sep 15;56(18):2475-2479. doi: 10.2169/internalmedicine.8571-16. Epub 2017 Aug 21. Intern Med. 2017. PMID: 28824070 Free PMC article. - Long-term outcome of anti-glomerular basement membrane antibody disease treated with plasma exchange and immunosuppression.
Levy JB, Turner AN, Rees AJ, Pusey CD. Levy JB, et al. Ann Intern Med. 2001 Jun 5;134(11):1033-42. doi: 10.7326/0003-4819-134-11-200106050-00009. Ann Intern Med. 2001. PMID: 11388816 - Predicting Outcome in Patients with Anti-GBM Glomerulonephritis.
van Daalen EE, Jennette JC, McAdoo SP, Pusey CD, Alba MA, Poulton CJ, Wolterbeek R, Nguyen TQ, Goldschmeding R, Alchi B, Griffiths M, de Zoysa JR, Vincent B, Bruijn JA, Bajema IM. van Daalen EE, et al. Clin J Am Soc Nephrol. 2018 Jan 6;13(1):63-72. doi: 10.2215/CJN.04290417. Epub 2017 Nov 21. Clin J Am Soc Nephrol. 2018. PMID: 29162595 Free PMC article. - Anti-glomerular basement membrane antibody disease treated with rituximab: A case-based review.
Syeda UA, Singer NG, Magrey M. Syeda UA, et al. Semin Arthritis Rheum. 2013 Jun;42(6):567-72. doi: 10.1016/j.semarthrit.2012.10.007. Epub 2013 Jan 24. Semin Arthritis Rheum. 2013. PMID: 23352254 Review. - Goodpasture's disease: a report of ten cases and a review of the literature.
Dammacco F, Battaglia S, Gesualdo L, Racanelli V. Dammacco F, et al. Autoimmun Rev. 2013 Sep;12(11):1101-8. doi: 10.1016/j.autrev.2013.06.014. Epub 2013 Jun 24. Autoimmun Rev. 2013. PMID: 23806563 Review.
Cited by
- Protein A immunoadsorption for anti-glomerular basement membrane nephritis.
Liu C, Li X, Yang Y, Zhao Y, Zhang L. Liu C, et al. Heliyon. 2024 Jul 23;10(15):e35049. doi: 10.1016/j.heliyon.2024.e35049. eCollection 2024 Aug 15. Heliyon. 2024. PMID: 39157406 Free PMC article. - Effectiveness and safety of immunoadsorption as a rescue treatment of inflammatory myopathies: report of three cases and literature review.
Nies JF, Hendrix C, Bartram MP, Spear R, Hagmann H, Benzing T, Kubacki T. Nies JF, et al. Ther Adv Musculoskelet Dis. 2024 May 17;16:1759720X241250238. doi: 10.1177/1759720X241250238. eCollection 2024. Ther Adv Musculoskelet Dis. 2024. PMID: 38764488 Free PMC article. - Clinical Profile and Renal Survival of Anti-Glomerular Basement Membrane Disease Patients: A Retrospective Case Series from Northern India.
Kumar A, Gupta S, Jarial KDS, Sangha S, Chauhan A, Sharma V, Sandal R, Sharma D. Kumar A, et al. Glomerular Dis. 2023 Oct 17;3(1):241-247. doi: 10.1159/000534498. eCollection 2023 Jan-Dec. Glomerular Dis. 2023. PMID: 38021463 Free PMC article. - Unleashing the power of complement activation: unraveling renal damage in human anti-glomerular basement membrane disease.
Tang A, Zhao X, Tao T, Xie D, Xu B, Huang Y, Li M. Tang A, et al. Front Immunol. 2023 Sep 15;14:1229806. doi: 10.3389/fimmu.2023.1229806. eCollection 2023. Front Immunol. 2023. PMID: 37781380 Free PMC article. Review. - Using imlifidase to elucidate the characteristics and importance of anti-GBM antibodies produced after start of treatment.
Tyrberg L, Andersson F, Uhlin F, Hellmark T, Segelmark M. Tyrberg L, et al. Nephrol Dial Transplant. 2023 Dec 20;39(1):45-54. doi: 10.1093/ndt/gfad132. Nephrol Dial Transplant. 2023. PMID: 37385828 Free PMC article.
References
- Couser WG (1988) Rapidly progressive glomerulonephritis: classification, pathogenetic mechanisms, and therapy. Am J Kidney Dis 11: 449–464. - PubMed
- Andrassy K, Küster S, Waldherr R, Ritz E (1991) Rapidly progressive glomerulonephritis: analysis of prevalence and clinical course. Nephron 59: 206–212. - PubMed
- Heaf J, Løkkegaard H, Larsen S (1999) The epidemiology and prognosis of glomerulonephritis in Denmark 1985–1997. Nephrol Dial Transplant 14: 1889–1897. - PubMed
- Bolton WK (1996) Goodpasture’s syndrome. Kidney Int 50: 1753–1766. - PubMed
MeSH terms
Substances
Grants and funding
The authors have no support or funding to report.
LinkOut - more resources
Full Text Sources
Other Literature Sources