Progesterone and nestorone promote myelin regeneration in chronic demyelinating lesions of corpus callosum and cerebral cortex - PubMed (original) (raw)
Progesterone and nestorone promote myelin regeneration in chronic demyelinating lesions of corpus callosum and cerebral cortex
Martine El-Etr et al. Glia. 2015 Jan.
Abstract
Multiple Sclerosis affects mainly women and consists in intermittent or chronic damages to the myelin sheaths, focal inflammation, and axonal degeneration. Current therapies are limited to immunomodulators and antiinflammatory drugs, but there is no efficient treatment for stimulating the endogenous capacity of myelin repair. Progesterone and synthetic progestins have been shown in animal models of demyelination to attenuate myelin loss, reduce clinical symptoms severity, modulate inflammatory responses and partially reverse the age-dependent decline in remyelination. Moreover, progesterone has been demonstrated to promote myelin formation in organotypic cultures of cerebellar slices. In the present study, we show that progesterone and the synthetic 19-nor-progesterone derivative Nestorone® promote the repair of severe chronic demyelinating lesions induced by feeding cuprizone to female mice for up to 12 weeks. Progesterone and Nestorone increase the density of NG2(+) oligodendrocyte progenitor cells and CA II(+) mature oligodendrocytes and enhance the formation of myelin basic protein (MBP)- and proteolipid protein (PLP)-immunoreactive myelin. However, while demyelination in response to cuprizone was less marked in corpus callosum than in cerebral cortex, remyelination appeared earlier in the former. The remyelinating effect of progesterone was progesterone receptor (PR)-dependent, as it was absent in PR-knockout mice. Progesterone and Nestorone also decreased (but did not suppress) neuroinflammatory responses, specifically astrocyte and microglial cell activation. Therefore, some progestogens are promising therapeutic candidates for promoting the regeneration of myelin.
Keywords: cuprizone; oligodendrocytes; progesterone receptor; progestogens; remyelination.
© 2014 Wiley Periodicals, Inc.
Conflict of interest statement
The authors have no conflict of interest.
Figures
Figure 1
Effects of a 3-week treatment with progesterone on MBP immunostaining and mRNA expression in the body of the corpus callosum. (A) Following cuprizone intoxication during 12 weeks, intact female mice received for 3 weeks a subcutaneous Silastic implant which was empty or filled with progesterone (P). MBP immunostaining and expression were compared to cuprizone-intoxicated and further untreated mice (Cup) or to control female mice, which were not exposed to cuprizone (Ctrl). Cuprizone induced a 50% demyelination, and progesterone treatment restored MBP+ myelin (n = 10 per group, corresponding to the results of 2 independent experiments. (B) MBP mRNA was measured in tissue blocks including corpus callosum and surrounding brain regions. Results were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH)) mRNA. Levels of MBP mRNA were decreased in response to cuprizone, and progesterone treatment reversed the effect (n = 4 per group). All data are presented as means ± S.E.M. and were analyzed by one-way ANOVA followed by Bonferroni tests. (* p < 0.05, **p <0.01, ***p < 0.001).
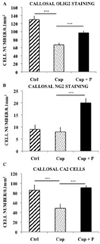
Figure 2
Effects of a 3-week treatment with progesterone after a 12-week cuprizone (Cup) intoxication on the number of oligodendroglial cells in the body of the corpus callosum of intact female mice. (A) Progesterone (P) enhanced the number of Olig2+ cells of the oligodendroglial lineage. Results were compared to cuprizone-intoxicated/further untreated mice (Cup) or control female mice, which were not exposed to cuprizone (Ctrl) (n = 6 per group). (B) Progesterone increased the number of NG2+ oligodendrocyte precursor cells (n = 5 per group). (C) Progesterone therapy also enhanced the number of mature CA II+ oligodendrocytes (n = 7 per group). All data are presented as means ± S.E.M. and were analyzed by one-way ANOVA followed by Bonferroni tests (***p < 0.001).
Figure 3
The intracellular progesterone receptor (PR) is necessary for the remyelinating effect of progesterone. Whereas treatment during 3 weeks with progesterone (P) was sufficient to restore MBP+ myelin within the corpus callosum of castrated wild-type mice (PR+/+) after 12 weeks of cuprizone (Cup), no remyelination was observed in ovariectomized PR knockout mice (PR−/−). Remyelination was only partial in castrated heterozygous mice (PR+/−). Results were compared to cuprizone-intoxicated/further untreated mice (Cup) or control female mice, which were not exposed to cuprizone (Ctrl) (n = 5 per group). All data are presented as means ± S.E.M. and were analyzed by two-way ANOVA (genotype × treatment) followed by Bonferroni tests (*p <0.01, *** p < 0.001).
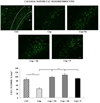
Figure 4
Nestorone promoted replenishment of the corpus callosum with mature oligodendrocytes. The administration of cuprizone (Cup) during 12 weeks induced a marked decrease in the number of CA II+ oligodendrocytes within the corpus callosum of intact female mice, when compared to controls, which were not exposed to cuprizone (Ctrl). Treatment during 3 weeks with 6 µg/day (N6) or 8 µg/day (N8) of Nestorone released by osmotic minipumps or Silastic implants of progesterone (P) restored the number of oligodendrocytes (n = 5–7 per group). The body of the corpus callosum is highlited by the white dotted lines. Cx: cortex; CC: corpus callosum; hi: hippocampus; v: lateral ventricule. All data are presented as means ± S.E.M. and were analyzed by one-way ANOVA followed by Bonferroni tests (***p < 0.001). Scale bar = 50 µm.
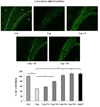
Figure 5
Nestorone stimulated the regeneration of MBP+ myelin in corpus callosum. The administration of cuprizone (Cup) during 12 weeks induced a marked decrease in the number of CA II+ oligodendrocytes within the corpus callosum of intact female mice, when compared to controls, which were not exposed to cuprizone (Ctrl). The remyelinating effect of Nestorone was weak when administered for 3 weeks at the dose of 4 µg/day (N4). MBP+ myelin was comparable to Ctrl or progesterone-treated mice (P) for doses of 6 µg/day (N6) or 8 µg/day (N8) of Nestorone (n = 6–8 per group). The body of the corpus callosum is highlited by the white dotted lines.. Cx: cortex; CC: corpus callosum; hi: hippocampus; v: lateral ventricule. All data are presented as means ± S.E.M. and were analyzed by one-way ANOVA followed by Bonferroni tests (**p < 0.01, ***p < 0.001). Scale bar = 50 µm.
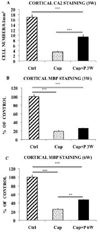
Figure 6
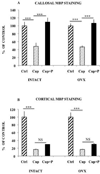
Replenishment of oligodendrocytes and remyelination in the cerebral cortex of intact female mice after 12 weeks of cuprizone intoxication. (A) Treatment with progesterone during 3 weeks (P 3W) enhanced the number of CA II+ oligodendrocytes. Results were compared to cuprizone-intoxicated/further untreated mice (Cup) or to control female mice, which were not exposed to cuprizone (Ctrl). (B) Cuprizone induced a 80% demyelination, and progesterone treatment during 3 weeks (P 3W) failed to significantly increase MBP immunostaining. (C) Treatment with progesterone during 6 weeks (P 6W) increased MBP immunostaining. All data are presented as means ± S.E.M. (n = 6–8 per group) and were analyzed by one-way ANOVA followed by Bonferroni tests (**p < 0.01, ***p < 0.001).
Figure 7
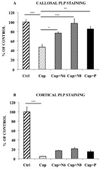
PLP immunostaining confirmed data obtained with MBP antibody: indeed Progesterone and Nestorone administered for 3 weeks after the end of a 12-week demyelination favor myelin repair in the corpus callosum (A) but not in the cortex (B), as shown by the enhancement of callosal (but not cortical) PLP immunostaining. All data are presented as means ± S.E.M. (n = 4–7 per group of intact female mice) and were analyzed by one-way ANOVA followed by Bonferroni tests (* p < 0.05, **p < 0.01, ***p < 0.001).
Figure 8
Progesterone and Nestorone administered during 3 weeks restored MBP+ myelin in the corpus callosum but not in the cerebral cortex of either intact or ovariectomized female mice. The administration of cuprizone (Cup) during 12 weeks caused a 50% and 80% decrease in MBP immunostaining within the corpus callosum and cerebral cortex, respectively. Cuprizone-induced demyelination was comparable between intact and ovariectomized females. Treatment with progesterone (P) during 3 weeks was sufficient to stimulate the regeneration of MBP+ myelin in the corpus callosum, whatever the presence of ovaries, but failed to significantly increase MBP immunostaining in the cerebral cortex. Results were compared to control female mice, which had not been exposed to cuprizone (Ctrl) (n = 6–8 per group). All data are presented as means ± S.E.M. and were analyzed by one-way ANOVA followed by Bonferroni tests (***p < 0.001, NS = non significant).
Figure 9
Progesterone and Nestorone blunted the increase in reactive GFAP+ astrocytes (A–B) and Iba1+ microglial cells (C–D) in both corpus callosum and cerebral cortex of intact female mice. After 12 weeks of cuprizone (Cup) intoxication, the numbers of GFAP+ astrocytes and Iba1+ microglial cells were markedly increased. Following the removal of cuprizone from the diet, treatment during 3 weeks with 8 µg/day of Nestorone (N8) via mini pumps or Silastic progesterone implants (P), reduced but not abolished) the increase of astrocytes in both structures. It also decreased the number of microglial cells by 50% in the corpus callosum (n = 6–8 per group) and nearly to control levels in the cortex. All data are presented as means ± S.E.M. and were analyzed by one-way ANOVA followed by Bonferroni tests (***p < 0.001).
Similar articles
- Progesterone and Nestorone facilitate axon remyelination: a role for progesterone receptors.
Hussain R, El-Etr M, Gaci O, Rakotomamonjy J, Macklin WB, Kumar N, Sitruk-Ware R, Schumacher M, Ghoumari AM. Hussain R, et al. Endocrinology. 2011 Oct;152(10):3820-31. doi: 10.1210/en.2011-1219. Epub 2011 Aug 9. Endocrinology. 2011. PMID: 21828184 Free PMC article. - The neural androgen receptor: a therapeutic target for myelin repair in chronic demyelination.
Hussain R, Ghoumari AM, Bielecki B, Steibel J, Boehm N, Liere P, Macklin WB, Kumar N, Habert R, Mhaouty-Kodja S, Tronche F, Sitruk-Ware R, Schumacher M, Ghandour MS. Hussain R, et al. Brain. 2013 Jan;136(Pt 1):132-46. doi: 10.1093/brain/aws284. Brain. 2013. PMID: 23365095 Free PMC article. - rHIgM22 enhances remyelination in the brain of the cuprizone mouse model of demyelination.
Mullin AP, Cui C, Wang Y, Wang J, Troy E, Caggiano AO, Parry TJ, Colburn RW, Pavlopoulos E. Mullin AP, et al. Neurobiol Dis. 2017 Sep;105:142-155. doi: 10.1016/j.nbd.2017.05.015. Epub 2017 May 30. Neurobiol Dis. 2017. PMID: 28576706 - How to Use the Cuprizone Model to Study De- and Remyelination.
Kipp M. Kipp M. Int J Mol Sci. 2024 Jan 24;25(3):1445. doi: 10.3390/ijms25031445. Int J Mol Sci. 2024. PMID: 38338724 Free PMC article. Review. - Five Decades of Cuprizone, an Updated Model to Replicate Demyelinating Diseases.
Vega-Riquer JM, Mendez-Victoriano G, Morales-Luckie RA, Gonzalez-Perez O. Vega-Riquer JM, et al. Curr Neuropharmacol. 2019;17(2):129-141. doi: 10.2174/1570159X15666170717120343. Curr Neuropharmacol. 2019. PMID: 28714395 Free PMC article. Review.
Cited by
- The mutual effect of progesterone and vitamin D in an animal model of peripheral nerve injury.
Nasirzadeh S, Hamidi GA, Banafshe HR, Tehrani MN, Shabani M, Abed A. Nasirzadeh S, et al. Res Pharm Sci. 2024 Aug 19;19(4):415-424. doi: 10.4103/RPS.RPS_18_23. eCollection 2024 Aug. Res Pharm Sci. 2024. PMID: 39399728 Free PMC article. - The Effects of Neuroactive Steroids on Myelin in Health and Disease.
Kalakh S, Mouihate A. Kalakh S, et al. Med Princ Pract. 2024;33(3):198-214. doi: 10.1159/000537794. Epub 2024 Feb 13. Med Princ Pract. 2024. PMID: 38350432 Free PMC article. Review. - Impact of endocrine dysregulation on disability and non-motor symptoms in pediatric onset multiple sclerosis.
Abe J, Jafarpour S, Vu MH, O'Brien D, Boyd NK, Vogel BN, Nguyen L, Paulsen KC, Saucier LE, Ahsan N, Mitchell WG, Santoro JD. Abe J, et al. Front Neurol. 2023 Dec 7;14:1304610. doi: 10.3389/fneur.2023.1304610. eCollection 2023. Front Neurol. 2023. PMID: 38130835 Free PMC article. - Progesterone attenuates Th17-cell pathogenicity in autoimmune uveitis via Id2/Pim1 axis.
Liu X, Gu C, Lv J, Jiang Q, Ding W, Huang Z, Liu Y, Su Y, Zhang C, Xu Z, Wang X, Su W. Liu X, et al. J Neuroinflammation. 2023 Jun 21;20(1):144. doi: 10.1186/s12974-023-02829-3. J Neuroinflammation. 2023. PMID: 37344856 Free PMC article. - Evaluations of memory, anxiety, and the growth factor IGF-1R after post-surgical menopause treatment with a highly selective progestin.
Bernaud VE, Koebele SV, Northup-Smith SN, Willeman MN, Barker C, Schatzki-Lumpkin A, Sanchez MV, Bimonte-Nelson HA. Bernaud VE, et al. Behav Brain Res. 2023 Jun 25;448:114442. doi: 10.1016/j.bbr.2023.114442. Epub 2023 Apr 20. Behav Brain Res. 2023. PMID: 37085118 Free PMC article.
References
- Acs P, Kipp M, Norkute A, Johann S, Clarner T, Braun A, Berente Z, Komoly S, Beyer C. 17beta-estradiol and progesterone prevent cuprizone provoked demyelination of corpus callosum in male mice. Glia. 2009;57:807–814. - PubMed
- Bastida CM, Tejada F, Cremades A, Penafiel R. The preovulatory rise of ovarian ornithine decarboxylase is required for progesterone secretion by the corpus luteum. Biochem Biophys Res Commun. 2002;293:106–111. - PubMed
- Blaustein JD, Turcotte JC. Estrogen receptor-immunostaining of neuronal cytoplasmic processes as well as cell nuclei in guinea pig brain. Brain Res. 1989;495:75–82. - PubMed
- Calabrese M, Filippi M, Gallo P. Cortical lesions in multiple sclerosis. Nat Rev Neurol. 2010;6:438–444. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous