The Agr quorum-sensing system regulates fibronectin binding but not hemolysis in the absence of a functional electron transport chain - PubMed (original) (raw)
The Agr quorum-sensing system regulates fibronectin binding but not hemolysis in the absence of a functional electron transport chain
Vera Pader et al. Infect Immun. 2014 Oct.
Abstract
Staphylococcus aureus is responsible for numerous chronic and recurrent infections, which are frequently associated with the emergence of small-colony variants (SCVs) that lack a functional electron transport chain. SCVs exhibit enhanced expression of fibronectin-binding protein (FnBP) and greatly reduced hemolysin production, although the basis for this is unclear. One hypothesis is that these phenotypes are a consequence of the reduced Agr activity of SCVs, while an alternative is that the lack of a functional electron transport chain and the resulting reduction in ATP production are responsible. Disruption of the electron transport chain of S. aureus genetically (hemB and menD) or chemically, using 2-n-heptyl-4-hydroxyquinoline N-oxide (HQNO), inhibited both growth and Agr activity and conferred an SCV phenotype. Supplementation of the culture medium with synthetic autoinducing peptide (sAIP) significantly increased Agr expression in both hemB mutant strains and S. aureus grown with HQNO and significantly reduced staphylococcal adhesion to fibronectin. However, sAIP did not promote hemolysin expression in hemB mutant strains or S. aureus grown with HQNO. Therefore, while Agr regulates fibronectin binding in SCVs, it cannot promote hemolysin production in the absence of a functional electron transport chain.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures
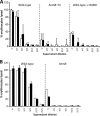
FIG 1
Loss of the electron transport chain reduces growth and Agr expression. Shown are growth (A and D), P3 expression (B and E), and P3 expression corrected for growth (C and F) of S. aureus SH1000 (A to C) and USA300 (D to F) and derived strains. (A to C) SH1000 grown in the absence or presence of HQNO and a hemB::Tn mutant in TSB as determined by the OD600 (growth) and A_520 (GFP fluorescence) measurements. (D to F) USA300 wild type, Δ_hemB, and Δ_menD_ grown in TSB. The measurements are as described for panels A to C. The data represent the means of at least 4 independent experiments, each in triplicate. The error bars represent the standard deviations of the mean. RFU, relative fluorescence units.
FIG 2
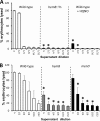
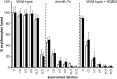
Loss of the electron transport chain results in enhanced fibronectin binding. (A) Attachment of wild-type (WT) S. aureus SH1000 grown in the absence or presence (+HQ) of HQNO and a hemB::Tn mutant (hemB) to immobilized human fibronectin. Also shown are values relating to the attachment of agr_-defective SH1001 (Δ_agr) grown in the absence or presence of HQNO and of a hemB Δ_agr_ strain. (B) Attachment of WT USA300 and hemB and menD mutants. The values represent the mean averages of at least 4 experiments done in triplicate, and the error bars represent the standard deviations of the mean. Values that are significantly greater (P = 0.05 by Student's t test) than that of the wild type are indicated by asterisks.
FIG 3
Loss of the electron transport chain results in significantly reduced hemolytic activity. Shown are hemolytic activities of culture supernatants of wild-type S. aureus SH1000 and derived strains (A) and USA300 and derived mutants (B). Culture supernatants were used undiluted (U) or after serial 2-fold dilutions, as indicated. The data represent the means of at least 4 independent experiments, each in triplicate. The error bars represent the standard deviations of the mean. Values that are significantly different (P < 0.05 by Student's t test) from those of the wild type at equivalent dilutions are indicated by asterisks.
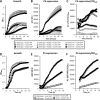
FIG 4
Synthetic AIP enhances agr expression in the absence of a functional electron transport chain. Shown are growth (A and D), P3 expression (B and E), and P3 expression corrected for growth (C and F) of SH1000 and derived strains (A to C) and USA300 and derived strains (D to F). (A to C) Wild-type S. aureus grown in the absence or presence of HQNO and that of a hemB::Tn mutant in TSB only or TSB supplemented with 1 μM or 10 μM sAIP. (D to F) Wild-type S. aureus and a hemB mutant grown in the absence or presence of 10 μM sAIP. The data represent the means of at least 4 independent experiments, each in triplicate. The error bars were omitted to enhance clarity but were all within 5% of the mean.
FIG 5
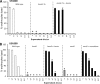
Agr regulates fibronectin binding in both the presence and absence of the electron transport chain. (A) Fibronectin binding of wild-type S. aureus SH1000 grown in the absence (WT) or presence (WT + HQ) of HQNO and a hemB::Tn mutant (hemB). Also shown are the fibronectin-binding levels of agr_-deficient mutants grown in the absence (Δ_agr) or presence (Δ_agr_ + HQ) of HQNO or in the hemB::Tn mutant background (Δ_agr hemB_). The culture medium consisted of TSB only or TSB supplemented with 1 μM sAIP or 10 μM sAIP. (B) Fibronectin binding of wild-type S. aureus USA300 and a hemB mutant grown in the absence or presence of 10 μM sAIP. Experiments were repeated 4 times in triplicate. Values that are significantly different (P < 0.05 by Student's t test) from those seen in the absence of sAIP are indicated by asterisks.
FIG 6
Agr activation by sAIP does not promote hemolytic activity in the absence of a functional electron transport chain. (A) Hemolytic activities of culture supernatants of wild-type SH1000 grown in the absence or presence of HQNO and of a hemB::Tn mutant in the absence (open bars) or presence (filled bars) of 10 μM sAIP. (B) Hemolytic activities of culture supernatants of wild-type USA300 and a hemB mutant in the absence (open bars) or presence (filled bars) of 10 μM sAIP. The data represent the mean averages of 4 experiments performed in triplicate, and the error bars represent the standard deviations of the mean. None of the values obtained from cultures containing sAIP were significantly different from those without sAIP at identical dilutions.
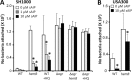
FIG 7
Agr activation by sAIP does not promote hemolytic activity in concentrated cultures in the absence of a functional electron transport chain. Wild-type SH1000 and a hemB::Tn mutant were concentrated to equivalent bacterial densities and cultured in TSB in the absence or presence of HQNO and the absence (open bars) or presence (filled bars) of 10 μM sAIP. The data represent the mean averages of 4 experiments performed in triplicate, and the error bars represent the standard deviations of the mean. None of the values obtained from cultures containing sAIP were significantly different from those without sAIP at identical dilutions.
FIG 8
Hemin and menadione restore growth and Agr activity in hemB and menD mutants, respectively. (A, B, D, and E) SH1000 hemB::Tn (A and D) and USA300 hemB (B and E) were grown in the absence or presence of 1 μg ml−1 hemin, and growth (A and B) was measured via OD600 readings, while P3 activity (corrected for OD600 values) was measured to determine Agr activity (D and E). (C and F) USA300 menD was grown in the absence or presence of 1 μg ml−1 menadione, and growth (C) and P3 activity (F) were measured as described for the hemB mutants. The data represent the means of at least 4 independent experiments, each in triplicate. The error bars were omitted to enhance clarity but were all within 5% of the mean.
FIG 9
Hemin and menadione restore hemolytic activity in hemB and menD mutants, respectively. (A) SH1000 hemB::Tn was grown in the absence or presence of 1 μg ml−1 hemin, and the hemolytic activity of the culture supernatant was determined. Culture supernatant from wild-type SH1000 is included for comparison. (B) USA300 hemB and menD were grown in the absence or presence of 1 μg ml−1 hemin or menadione, and the hemolytic activity of the culture supernatant was determined. Culture supernatant from wild-type USA300 is included for comparison. The data represent the means of 4 experiments done in triplicate, and the error bars represent the standard deviations of the mean. Values that are significantly different (P < 0.05 by Student's t test) from those of the wild type at the same dilution are indicated by asterisks.
Similar articles
- Increased expression of clumping factor and fibronectin-binding proteins by hemB mutants of Staphylococcus aureus expressing small colony variant phenotypes.
Vaudaux P, Francois P, Bisognano C, Kelley WL, Lew DP, Schrenzel J, Proctor RA, McNamara PJ, Peters G, Von Eiff C. Vaudaux P, et al. Infect Immun. 2002 Oct;70(10):5428-37. doi: 10.1128/IAI.70.10.5428-5437.2002. Infect Immun. 2002. PMID: 12228267 Free PMC article. - Eliminating extracellular autoinducing peptide signals inhibits the Staphylococcus aureus quorum sensing agr system.
Inagaki R, Koshiba A, Nasuno E, Kato N. Inagaki R, et al. Biochem Biophys Res Commun. 2024 Jun 4;711:149912. doi: 10.1016/j.bbrc.2024.149912. Epub 2024 Apr 7. Biochem Biophys Res Commun. 2024. PMID: 38615572 - Staphylococcus aureus sigma B-dependent emergence of small-colony variants and biofilm production following exposure to Pseudomonas aeruginosa 4-hydroxy-2-heptylquinoline-N-oxide.
Mitchell G, Séguin DL, Asselin AE, Déziel E, Cantin AM, Frost EH, Michaud S, Malouin F. Mitchell G, et al. BMC Microbiol. 2010 Jan 30;10:33. doi: 10.1186/1471-2180-10-33. BMC Microbiol. 2010. PMID: 20113519 Free PMC article. - Staphylococcus aureus Small Colony Variants (SCVs): a road map for the metabolic pathways involved in persistent infections.
Proctor RA, Kriegeskorte A, Kahl BC, Becker K, Löffler B, Peters G. Proctor RA, et al. Front Cell Infect Microbiol. 2014 Jul 28;4:99. doi: 10.3389/fcimb.2014.00099. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 25120957 Free PMC article. Review. - Persistence of Staphylococcus aureus: Multiple Metabolic Pathways Impact the Expression of Virulence Factors in Small-Colony Variants (SCVs).
Tuchscherr L, Löffler B, Proctor RA. Tuchscherr L, et al. Front Microbiol. 2020 May 21;11:1028. doi: 10.3389/fmicb.2020.01028. eCollection 2020. Front Microbiol. 2020. PMID: 32508801 Free PMC article. Review.
Cited by
- Intracellular Staphylococcus aureus Modulates Host Central Carbon Metabolism To Activate Autophagy.
Bravo-Santano N, Ellis JK, Mateos LM, Calle Y, Keun HC, Behrends V, Letek M. Bravo-Santano N, et al. mSphere. 2018 Aug 8;3(4):e00374-18. doi: 10.1128/mSphere.00374-18. mSphere. 2018. PMID: 30089650 Free PMC article. - Klebsiella pneumoniae exhibiting a phenotypic hyper-splitting phenomenon including the formation of small colony variants.
Doğan E, Sydow K, Heiden SE, Eger E, Wassilew G, Proctor RA, Bohnert JA, Idelevich EA, Schaufler K, Becker K. Doğan E, et al. Front Cell Infect Microbiol. 2024 Mar 27;14:1372704. doi: 10.3389/fcimb.2024.1372704. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38601740 Free PMC article. - Naturally occurring polymorphisms in the virulence regulator Rsp modulate Staphylococcus aureus survival in blood and antibiotic susceptibility.
Krishna A, Holden MTG, Peacock SJ, Edwards AM, Wigneshweraraj S. Krishna A, et al. Microbiology (Reading). 2018 Sep;164(9):1189-1195. doi: 10.1099/mic.0.000695. Epub 2018 Jul 20. Microbiology (Reading). 2018. PMID: 30028663 Free PMC article. - Computational Mutagenesis and Inhibition of Staphylococcus aureus AgrA LytTR Domain Using Phenazine Scaffolds: Insight From a Biophysical Study.
Manu P, Nketia PB, Osei-Poku P, Kwarteng A. Manu P, et al. Biomed Res Int. 2024 Sep 18;2024:8843954. doi: 10.1155/2024/8843954. eCollection 2024. Biomed Res Int. 2024. PMID: 39328594 Free PMC article. - Accessory Gene Regulator (agr) Allelic Variants in Cognate Staphylococcus aureus Strain Display Similar Phenotypes.
Tan L, Huang Y, Shang W, Yang Y, Peng H, Hu Z, Wang Y, Rao Y, Hu Q, Rao X, Hu X, Li M, Chen K, Li S. Tan L, et al. Front Microbiol. 2022 Feb 25;13:700894. doi: 10.3389/fmicb.2022.700894. eCollection 2022. Front Microbiol. 2022. PMID: 35295312 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- 100958/WT_/Wellcome Trust/United Kingdom
- MR/J006874/1/MRC_/Medical Research Council/United Kingdom
- HHSN272200700055C/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources