Reversible H atom abstraction catalyzed by the radical S-adenosylmethionine enzyme HydG - PubMed (original) (raw)
. 2014 Sep 24;136(38):13086-9.
doi: 10.1021/ja504618y. Epub 2014 Sep 10.
Affiliations
- PMID: 25099480
- PMCID: PMC4183638
- DOI: 10.1021/ja504618y
Reversible H atom abstraction catalyzed by the radical S-adenosylmethionine enzyme HydG
Benjamin R Duffus et al. J Am Chem Soc. 2014.
Abstract
The organometallic H-cluster at the active site of [FeFe]-hydrogenases is synthesized by three accessory proteins, two of which are radical S-adenosylmethionine enzymes (HydE, HydG) and one of which is a GTPase (HydF). In this work we probed the specific role of H atom abstraction in HydG-catalyzed carbon monoxide and cyanide production from tyrosine. The isotope distributions of 5'-deoxyadenosine and p-cresol were evaluated using deuterium-labeled tyrosine substrates in H2O and D2O. The observation of multiply deuterated 5'-deoxyadenosine and deuterated S-adenosylmethionine when the reaction is carried out in D2O provides evidence for a 5'-deoxyadenosyl radical-mediated abstraction of a hydrogen atom from a solvent-exchangeable position as a reversible event.
Figures
Figure 1
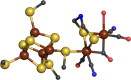
[FeFe]-hydrogenase H-cluster active site from Clostridium pasteurianum (PDB: 3C8Y).
Figure 2
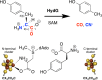
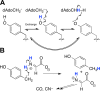
Reaction catalyzed by HydG. Top: Conversion of tyrosine to _p_-cresol, CO, and CN−. Concomitant with this reaction, SAM is converted to methionine and 5′-deoxyadenosine. Bottom: Two [4Fe-4S] clusters are involved. The N-terminal cluster binds and reductively cleaves SAM, and is required for the cleavage of tyrosine to produce _p_-cresol. The C-terminal cluster is important for diatomic ligand production, and the unique iron of this cluster may provide a site for diatomic ligand coordination prior to transfer to HydF. The cysteine motifs coordinating each cluster are shown below the cluster.
Figure 3
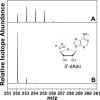
ESI-MS of HydG reaction product dAdoH for assays performed in 95% D2O buffer (50 mM tris, pD 8.1). (A) Full reaction. (B) dAdoH reference sample in H2O. Spectra are represented as normalized, extracted ion chromatograms.
Figure 4
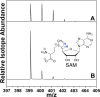
ESI-MS of SAM for HydG assays performed in 95% D2O buffer (50 mM tris, pD 8.1). (A) Full reaction. (B) SAM reference sample in H2O. Spectra are represented as normalized, extracted ion chromatograms.
Figure 5
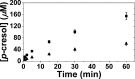
Quantitative _p_-cresol product detection in tris-H2O (■) and 95% tris-D2O (▲). Assays contained 40 μM HydG (9.5 ± 0.2 Fe/protein), 1 mM SAM, 1 mM Tyr, 5 mM dithionite, performed at 37 °C in 50 mM tris, 300 mM KCl, pH/pD 8.1.
Figure 6
Mechanistic proposals for observed HydG H atom abstraction—reabstraction events and dAdo• regeneration. Hydrogen atoms that have undergone exchange with solvent are colored blue.
Similar articles
- A Redox Active [2Fe-2S] Cluster on the Hydrogenase Maturase HydF.
Shepard EM, Byer AS, Betz JN, Peters JW, Broderick JB. Shepard EM, et al. Biochemistry. 2016 Jun 28;55(25):3514-27. doi: 10.1021/acs.biochem.6b00528. Epub 2016 Jun 14. Biochemistry. 2016. PMID: 27232385 Free PMC article. - [FeFe]-hydrogenase maturation: HydG-catalyzed synthesis of carbon monoxide.
Shepard EM, Duffus BR, George SJ, McGlynn SE, Challand MR, Swanson KD, Roach PL, Cramer SP, Peters JW, Broderick JB. Shepard EM, et al. J Am Chem Soc. 2010 Jul 14;132(27):9247-9. doi: 10.1021/ja1012273. J Am Chem Soc. 2010. PMID: 20565074 - H-cluster assembly intermediates built on HydF by the radical SAM enzymes HydE and HydG.
Byer AS, Shepard EM, Ratzloff MW, Betz JN, King PW, Broderick WE, Broderick JB. Byer AS, et al. J Biol Inorg Chem. 2019 Sep;24(6):783-792. doi: 10.1007/s00775-019-01709-7. Epub 2019 Sep 6. J Biol Inorg Chem. 2019. PMID: 31493152 - Overview of the Maturation Machinery of the H-Cluster of [FeFe]-Hydrogenases with a Focus on HydF.
Bortolus M, Costantini P, Doni D, Carbonera D. Bortolus M, et al. Int J Mol Sci. 2018 Oct 11;19(10):3118. doi: 10.3390/ijms19103118. Int J Mol Sci. 2018. PMID: 30314343 Free PMC article. Review. - A radical solution for the biosynthesis of the H-cluster of hydrogenase.
Peters JW, Szilagyi RK, Naumov A, Douglas T. Peters JW, et al. FEBS Lett. 2006 Jan 23;580(2):363-7. doi: 10.1016/j.febslet.2005.12.040. Epub 2005 Dec 22. FEBS Lett. 2006. PMID: 16386249 Review.
Cited by
- Biosynthetic versatility and coordinated action of 5'-deoxyadenosyl radicals in deazaflavin biosynthesis.
Philmus B, Decamps L, Berteau O, Begley TP. Philmus B, et al. J Am Chem Soc. 2015 Apr 29;137(16):5406-13. doi: 10.1021/ja513287k. Epub 2015 Apr 20. J Am Chem Soc. 2015. PMID: 25781338 Free PMC article. - A Redox Active [2Fe-2S] Cluster on the Hydrogenase Maturase HydF.
Shepard EM, Byer AS, Betz JN, Peters JW, Broderick JB. Shepard EM, et al. Biochemistry. 2016 Jun 28;55(25):3514-27. doi: 10.1021/acs.biochem.6b00528. Epub 2016 Jun 14. Biochemistry. 2016. PMID: 27232385 Free PMC article. - Biosynthesis of the [FeFe] Hydrogenase H Cluster: A Central Role for the Radical SAM Enzyme HydG.
Suess DL, Kuchenreuther JM, De La Paz L, Swartz JR, Britt RD. Suess DL, et al. Inorg Chem. 2016 Jan 19;55(2):478-87. doi: 10.1021/acs.inorgchem.5b02274. Epub 2015 Dec 24. Inorg Chem. 2016. PMID: 26703931 Free PMC article. - [FeFe]-hydrogenase maturation: insights into the role HydE plays in dithiomethylamine biosynthesis.
Betz JN, Boswell NW, Fugate CJ, Holliday GL, Akiva E, Scott AG, Babbitt PC, Peters JW, Shepard EM, Broderick JB. Betz JN, et al. Biochemistry. 2015 Mar 10;54(9):1807-18. doi: 10.1021/bi501205e. Epub 2015 Mar 2. Biochemistry. 2015. PMID: 25654171 Free PMC article. - Mechanism of Radical Initiation in the Radical S-Adenosyl-l-methionine Superfamily.
Broderick WE, Hoffman BM, Broderick JB. Broderick WE, et al. Acc Chem Res. 2018 Nov 20;51(11):2611-2619. doi: 10.1021/acs.accounts.8b00356. Epub 2018 Oct 15. Acc Chem Res. 2018. PMID: 30346729 Free PMC article. Review.
References
- Vignais P.; Billoud B. Chem. Rev. 2007, 107, 4206. - PubMed
- Peters J. W.; Lanzilotta W. N.; Lemon B. J.; Seefeldt L. C. Science 1998, 282, 1853. - PubMed
- McGlynn S. E.; Shepard E. M.; Winslow M. A.; Naumov A. V.; Duschene K. S.; Posewitz M. C.; Broderick W. E.; Broderick J. B.; Peters J. W. FEBS Lett. 2008, 582, 2183. - PubMed
- Posewitz M. C.; King P. W.; Smolinski S. L.; Zhang L.; Seibert M.; Ghirardi M. L. J. Biol. Chem. 2004, 279, 25711. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P20 RR-024237/RR/NCRR NIH HHS/United States
- P20 RR-020185/RR/NCRR NIH HHS/United States
- P20 RR-16455-08/RR/NCRR NIH HHS/United States
- P20 RR024237/RR/NCRR NIH HHS/United States
- P20 RR016455/RR/NCRR NIH HHS/United States
- P20 RR020185/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources